Effects of Two Halophytic Plants (Kochia and Atriplex) on Digestibility, Fermentation and Protein Synthesis by Ruminal Microbes Maintained in Continuous Culture
Article information
Abstract
Eight continuous culture fermenters were used in a completely randomized design to evaluate various nutritional values of Kochia (Kochia scoparia) compared with Atriplex (Atriplex dimorphostegia). Dried and pelleted samples (leaves and stems) provided substrate for metabolism by ruminal microbes maintained in a continuous culture fermentation system. Results indicated that there were no differences (p>0.05) in dry matter (DM) and crude protein (CP) digestibility between the two halophytic plants. Atriplex had higher (p<0.05) organic matter (OM) digestibility compared with Kochia. Neutral detergent fiber (aNDF) digestibility of Atriplex (411 g/kg) was higher (p<0.05) than that of Kochia (348 g/kg), however acid detergent fiber (ADF) digestibility was higher (p<0.05) in Kochia compared with Atriplex (406 vs. 234 g/kg). There were no differences (p>0.05) between the two halophytic plants in molar proportion of acetate and propionate, but the concentration of butyrate and valerate in Kochia were about two fold of Atriplex (p<0.05). When Kochia provided substrate to the microbes, protein synthesis was higher (p<0.05) compared with feeding Atriplex (5.96 vs. 4.85 g N/kg of OM truly digested). It was concluded that Kochia scoparia and Atriplex dimorphostegia had similar digestibility of DM and CP. It appears that these halophytic plants may not have enough digestible energy for high producing ruminants.
INTRODUCTION
Halophytic plants constitute a significant part of the local flora in arid and semiarid regions and in many cases represent supplementary or emergency feed during the prolonged seasons of drought. These forages are grazed by sheep, goats and camels and usually contain sufficient crude protein and (or) carbohydrate components to have significant nutritional potential. During the last two decades, there has been increased interest in planting halophytes in salty agricultural regions around the world (Riasi and Danesh Mesgaran, 2008). Kochia spp. and Atriplex spp. that belonging to the chenopodiaceae family, have considerable forage potential in the arid and semiarid rangelands of West Asia. General nutritional characteristics of these plants have been well defined (Gihad and El Shaer, 1992; El-Shatnawi and Turuk, 2002; Danesh Mesgaran and Stern, 2005; Riasi et al., 2008). However, more information on how these plants affect microbial fermentation in the rumen needs to be determined for them to be effectively used as a forage source for ruminant animals.
Dual flow continuous culture fermenters that simulate the ruminal environment have been used to measure feed digestion and microbial metabolism (Stern et al., 1997). In addition, continuous culture systems provide a means to evaluate the effect of nutrients on metabolism of microbes maintained under controlled pH, turnover rate and nutrient intake (Michalet-Doreau and Ould-Bah, 1992). Currently, there is only limited data for digestibility and ruminal fermentation parameters of halophytic plants using the in vitro methods. The objective of this experiment was to study the nutritive value of Kochia scoparia and Atriplex dimorphostegia using the continuous culture fermenter system.
MATERIAL AND METHODS
Halophytic plants and continuous culture study
Samples of Kochia scoparia and Atriplex dimorphostegia (stems and leaves) were harvested at mid bloom stage from the Salinity Research Station of Birjand University with clay-sandy soil (pH = 7.4 and EC = 10.3 dS/m) and salty irrigation water (pH = 6.8, EC = 5.5 dS/m, and Na+ = 24.7 meq/L). Samples of each plant were composited and sub-samples were taken from each composite and then dried in a forced air oven (65°C) for 48 h. Samples were ground using a Wiley mill (two-mm screen) and analyzed for total N (Kjeldahl method, Kjeltec 2300 Autoanalyzer, Foss Tecator AB, Hogans, Sweden), neutral detergent and acid detergent fiber (aNDF and ADF, respectively, Van Soest et al., 1991) and ash (AOAC, 2000, ID 942.05). Heat-stable α-amylase was used in the aNDF assay (Udén et al., 2005). Non-protein N was chemically determined using trichloroacetic acid 78 (1 g/100 ml) solution (Licitra et al., 1996).
Eight dual-flow continuous culture fermenters (Hannah et al., 1986) were inoculated with ruminal fluid (1,030 ml) from a Holstein cow. The cow was surgically fitted with a ruminal cannula made of soft plastic, housed in a well-ventilated barn, had free access to water, and was fed a total mixed diet that was formulated to meet its nutrient requirements (NRC, 2001). The diet consisted of 1.8 kg alfalfa hay, 9.5 kg corn silage, 0.7 kg wheat straw, and 4.4 kg concentrate mix on a DM basis. The concentrate mix was composed of 31% ground corn, 20% dried molasses, 16.5% soybean meal, 20% soybean hull, 1.5% yeast, 2% blood meal, and 9% vitamin-mineral mix. Ruminal fluid was collected using a vacuum pump approximately 2 h after the morning feeding (09:30), strained through four layers of cheese cloth, and anaerobically transported to the laboratory in a prewarmed insulated thermos.
The experimental period was 10 d in length, with seven d for adaptation followed by 3 d for sampling. Each fermenter was continuously infused with artificial saliva containing 0.5 g of urea/L (pH = 8.25). Solid and liquid dilution rates were adjusted daily to approximate 0.05 and 0.10 h−1 respectively, by regulation of saliva input and filtrate removal rates. Temperature of fermenter contents was maintained at 38.6°C±0.1 by an electrical heater and the pH at 6.5±0.5 by the addition of five N HCl or five N NaOH through automated peristaltic pumps that were controlled by an electronic data acquisition system (Daisy Lab®). Fermenters were constantly purged with N2 gas (40 ml/min) to preserve anaerobic condition. Fermenters were supplied daily with 75 g DM of pelleted Kochia scoparia or Atriplex dimorphostegia by an automated feeding mechanism adjusted to deliver the diet in eight equal portions over a 24-h period to establish steady-state conditions. Solid and liquid effluent weights were recorded daily at 09:00 and discarded until the last three days of the experiment. On the last 3 days, fermenter effluents were maintained at 2 to 4°C by a cold water bath to retard microbial metabolism. Total effluent for each 24 h of the 3-d sample period was recorded, combined and homogenized (Power Gen Model 700) and a 1-L sample was removed by vacuum aspiration and stored at −20°C for analysis. Fresh composite samples were used for total nitrogen (N), ammonia-nitrogen (NH3-N) and volatile fatty acid (VFA) analyses. Freeze-dried samples were ground through a 1-mm screen and used for all other analyses (DM, OM, ash, aNDF, ADF, and purine). Bacterial samples (fluid-associated bacteria) were collected from fermenter contents on the last sampling day by straining the total content of each fermenter through two layers of cheese cloth. This strained fluid was centrifuged at 1,000 g for 10 min to remove feed particles and any protozoa that may have been present. The supernatant was centrifuged at 20,000 g for 20 min to isolate bacterial cells. The resulting supernatant fluid was discarded and the precipitate (bacterial cells) was re-suspended in de-ionized water for freeze drying. Bacterial cells were used to determine the concentration of purines that were used as a bacterial marker.
Total N of effluent samples was determined by macro-Kjeldal procedure (AOAC, 2000) and the bacterial N was determined using a true spec nitrogen analyzer (True Spec Series Leco, USA). Concentration of NH3-N in effluent samples was determined by direct stem distillation using a Kjeltec 2300 Autoanalyzer (Foss Tecator AB, Hogans, Sweden). Purine concentration in effluent and bacterial cells was determined using the method of Zinn and Owens (1986). Purine content of effluent and bacteria was used to partition flow of effluent N into bacterial and dietary nitrogen. Effluent VFA concentration was measured by gas chromatography (Hewlett-Packard, model 5880A, Palo Alto, CA) with a Carbopack DA/0.3% Carbowax 20M column (Supelco, Bellefonte, PA).
Calculations and statistical analysis
Digestibility of nutrients was calculated using the following equations:
Bacterial N flow (g/d) was calculated directly from the total bacterial DM percentage obtained from the bacterial pellets isolated from culture contents and the mean flow rate of fermenter contents using the following equation:
Efficiency of bacterial synthesis was calculated as follows:
Results were analyzed using the GLM procedure of SAS (1998) as a completely randomized design with fermenter flasks as replicates. Statistical differences between the two halophytic plants were determined using Duncan’s multiple range test.
RESULTS AND DISCUSSION
Chemical composition and digestion
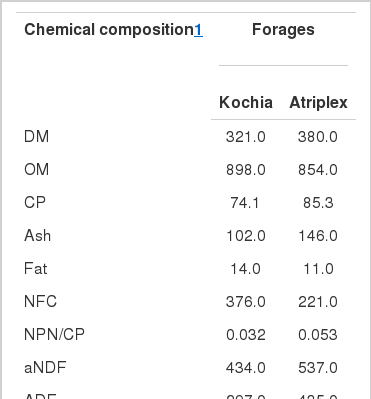
Some chemical composition of Kochia scoparia and Atriplex dimorphostegia is shown in Table 1. In agreement with Riasi et al. (2008) the DM, ash, aNDF and ADF content of Kochia was lower than Atriplex. However, there are some contradictions in chemical composition of halophytic forages between assays. In the present study CP content of Kochia was lower than some previous reports (Cohen et al., 1989; Madrid et al., 1996; Riasi et al., 2008). Differences among studies could be due to forage production condition, stage of harvesting, leaf:stem ratio or genetic variation (Gihad and El Shaer, 1992; Benjamin et al., 1995; El-Shatnawi and Abdullah, 2003). The NPN/CP ratio of Kochia and Atriplex (0.032 and 0.053, respectively) was relatively lower than the common forages such as alfalfa hay. This finding is important, because NPN content can influence CP quality of forages for ruminants (Cohen et al., 1989; Ben Salem et al., 2004).
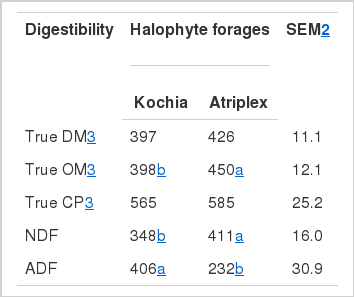
Nutrient digestibility of Kochia scoparia and Atriplex dimorphostegia is shown in Table 2. There was no difference (p>0.05) between true DM digestibility of Kochia compared with Atriplex. In general, DM digestibility determined using in vitro methods is lower than digestibility determined using in vivo and in situ methods (Stern et al., 1997). Consistent with this observation, true DM digestibility of Kochia (397 g/kg) and Atriplex (426 g/kg) (Table 2) was lower than DM digestibility of Kochia and Atriplex determined using an in situ procedure (444 and 472 g/kg, respectively) (Riasi et al., 2008).
Although the OM content of Kochia was higher than Atriplex (898 vs. 854 g/kg) (Table 1), Atriplex had higher true OM digestibility compared with Kochia. Ghadaki et al. (1975) determined nutritive values of introduced and native range plants grown under natural conditions in Iran and showed that the shrubs had higher percentages of lignin, and there was a relationship between their lignin content and in vitro true OM digestibility. However, it should be noted that moisture, pressure, and heat applied to continuous culture diets during pelleting may reduce the OM digestibility (Stern et al., 1997).
Kochia and Atriplex had about 50% true CP digestibility and there was no difference (p>0.05) between the two Halophytes. The CP digestibility of Kochia and Atriplex (Table 2) was higher than values reported by Danesh Mesgaran and Stern (2005). However, concordant with Danesh Mesgaran et al. (2004) and Riasi et al. (2008), there were no differences (p>0.05) between CP digestibilities of the two halophytic plants.
The aNDF digestibility of Atriplex was higher (p<0.05) than that of Kochia while ADF digestibility showed the opposite effect (p<0.05). These results may be related to chemical composition of plants regarding variations in attachments of hemicellulose to phenolic rings (Moore and Cherney, 1986) and/or a limitation in availability of certain nutrients such as valerate, branched-chain VFA and amino acids that are required by cellulolytic bacteria (Hoover, 1986; Hussein et al., 1991). In agreement with Mansfield et al. (1994) who observed a negative relationship between NFC concentration of diets and NDF digestibility values, our study showed that NFC concentrations of Kochia and Atriplex (376 and 221 g/kg, respectively) (Table 1) may have affected their NDF digestibility. A lower concentration of valerate with Atriplex (Table 3) might have affected its ADF digestibility (Table 2) (Hoover, 1986).
Volatile fatty acids
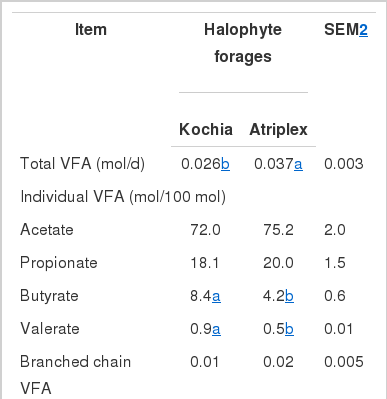
Total daily VFA concentration (mol/d) and molar proportions of the major VFA (acetate, propionate, butyrate, and valerate) are presented in Table 3. Total VFA concentration of Atriplex was higher (p<0.05) than that Kochia (0.037 vs. 0.026 mol/d). Mansfield et al. (1994) noted that in continuous culture fermenters, total VFA production decreased as RDP concentration in the diets decreased. In the current experiment, the two halophytic plants had similar CP digestion, so the higher total VFA concentration of Atriplex may be attributed to true OM digestibility (Table 2).
There were no differences (p>0.05) between the two halophytic plants in molar proportions of acetate and propionate. According to Mansfield at al. (1994) who showed that molar proportions of butyrate decreased and acetate increased as dietary NFC concentration decreased, molar proportion of butyrate was (p<0.05) lower with Atriplex (NFC = 221 g/kg) compared with Kochia (NFC = 376 g/kg). Mansfield at al. (1994) reported a switch from NFC to fiber fermentation when dietary NFC was reduced. The major source of variation affecting molar proportions of ruminal VFA is OM digestibility, so that feeding more digestible forages is associated with lower proportions of acetate and higher proportions of both propionate and butyrate (Lopez et al., 2000). However, our data showed in addition to higher OM digestibility of Atriplex its butyrate was lower than that Kochia. This finding may be attributed to factors that induce changes in various parameters of the rumen environment (osmotic pressure, redox potential, pH, and turnover rate). In the present study concentrations of valerate of Kochia (0.9 mol/100 mol) was higher (p<0.05) than that of Atriplex (0.5 mol/100 mol) and the concentration of branched chain VFA (BCVFA) was too low. Consistent with our data, other research indicated that decreasing dietary NFC (Mansfield et al., 1994) reduced concentration of either valerate and one or more of the branched-chain VFA.
Nitrogen metabolism
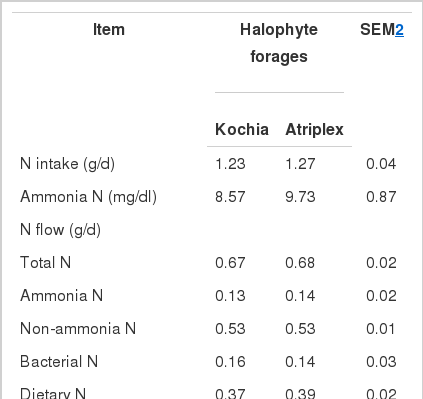
Nitrogen intake, NH3-N, N flow, and bacterial protein synthesis are presented in Table 4. No difference (p>0.05) was observed between Kochia and Atriplex in regard to NH3-N concentrations, total N flow, NH3-N flow, non NH3-N flow, bacterial N flow, and dietary N flow; however, efficiency of bacterial protein synthesis was higher (p<0.05) for Kochia compared with Atriplex (5.96 vs. 4.85 g of N/kg of OM truly digested).
A concentration of 5 mg/dl of NH3-N has been suggested as the minimum amount required to maximize efficiency of bacterial growth (Satter and Slyter, 1974). This value is lower than that obtained for Kochia and Atriplex (8.57 and 9.73 mg/dl, respectively) in the present study. Ariza et al. (2001) reported that NH3-N concentration in the fermenters depends on the extent of CP degradation and N uptake by ruminal bacteria. Fractions of N flow were not different (p>0.05) between the two halophytic forages which is probably due to the small difference between N intakes. In general, total N flow and its fractions for halophytic plants were lower than those previous reported for different diets using the continuous culture procedure (Hussein et al., 1991; Mansfield et al., 1994).
The lower efficiency of bacterial protein synthesis obtained with Atriplex was not a result of limited NH3-N, because its concentrations were about two fold that recommended by Satter and Slyter (1974) for maximum microbial growth to be achieved. Hence, other factors such as availability of small peptides and (or) certain amino acids may have been limiting when Atriplex was fed to the fermenters. It has been shown that an inverse relationship can exist between VFA and microbial biomass production (William, 2000). Consistent with this observation, when Atriplex was fed to the fermenters, total VFA yield (mol/d) was higher (Table 3) and efficiency of bacterial protein synthesis was lower (Table 4) than that of Kochia. It has been suggested that the type of carbohydrate and its rate of fermentation could influence efficiency when expressed as total synthesis of microbial protein per unit of gas produced (William, 2000).
IMPLICATIONS
Kochia scoparia and Atriplex dimorphostegia had similar digestibility of DM and CP, while the apparent and true OM, NDF and ADF digestibilities of these halophytic plants differed. Considering that VFA concentration of Kochia scoparia and Atriplex dimorphostegia was relatively low, it appears that these halophytic plants may not have enough digestible energy for high producing ruminants. It seems that lower dietary NFC in Atriplex compared with Kochia reduced its concentration of valerate. Degradability of CP from Kochia scoparia and Atriplex dimorphostegia seemed to achieve a concentration of NH3-N that maximized bacterial growth (>5 mg/dl), however efficiency of bacterial protein synthesis differed between the two halophytic plants and the reason for this observation is unclear.
ACKNOWLEDGEMENTS
This work was conducted with the financial support of the University of Minnesota, St. Paul, USA and Ferdowsi University of Mashad, Iran.