 |
 |
| Anim Biosci > Volume 28(12); 2015 > Article |
|
Abstract
Milk lysozyme is the ubiquitous enzyme in milk of mammals. In this study, the cDNA sequence of a new chicken-type (c-type) milk lysozyme gene (YML), was cloned from yak mammary gland tissue. A 444 bp open reading frames, which encodes 148 amino acids (16.54 kDa) with a signal peptide of 18 amino acids, was sequenced. Further analysis indicated that the nucleic acid and amino acid sequences identities between yak and cow milk lysozyme were 89.04% and 80.41%, respectively. Recombinant yak milk lysozyme (rYML) was produced by Escherichia coli BL21 and Pichia pastoris X33. The highest lysozyme activity was detected for heterologous protein rYML5 (M = 1,864.24 U/mg, SD = 25.75) which was expressed in P. pastoris with expression vector pPICZαA and it clearly inhibited growth of Staphylococcus aureus. Result of the YML gene expression using quantitative polymerase chain reaction showed that the YML gene was up-regulated to maximum at 30 day postpartum, that is, comparatively high YML can be found in initial milk production. The phylogenetic tree indicated that the amino acid sequence was similar to cow kidney lysozyme, which implied that the YML may have diverged from a different ancestor gene such as cow mammary glands. In our study, we suggest that YML be a new c-type lysozyme expressed in yak mammary glands that plays a role as host immunity.
Lysozyme is a kind of N-acetylmuramide-glycanohydrolase which is ubiquitous in plants, animals, and microorganisms. Three major distinct types of lysozyme have been identified: chicken-type (c-type), invertebrate-type (i-type), and goose-type (g-type) lysozyme (Callewaert and Michiels, 2010). As a natural non-specific defense factor in humans and animals, lysozyme can destroy the cell walls of Gram positive bacteria by hydrolyzing the link of β-1,4 glycosidic bonds between the peptidoglycan N-acetyl glucosamine and the N-acetylmuramic acid, thus damaging the bracket of the peptidoglycan causing the cells to disintegrate by internal osmotic pressure (Irwin, 2004; Corin et al., 2012; Kastorna et al., 2012).
Due to its biological characteristics, lysozymes have been applied in various industry fields. Under natural conditions, most of the lysozymes maintain their stable chemical properties. Even though lysozymes experience dramatically changes in the pH range of 1.2 to 11.3, they can keep their enzyme structure and activity (Hannig et al., 2011; Wilken and Nikolov, 2011). Lysozymes also have antibacterial activities without other adverse reactions or side effects (Marsh and Rice, 2010; Lan et al., 2012; Ng et al., 2012). So the development of lysozyme as clinical product seems to be beneficial to the health of animals (Brundige et al., 2010). At the same time, bio-engineering research on lysozymes offer benefits to the food industry (Proctor and Cunningham, 1988). For humans, one of the main non-specific host defense factors is accomplished through lysozyme in milk. Research related to the host defense of human and bovine milk shows that there are 268 proteins in human and 269 proteins in bovine. Therefore, milk provides not only as nutrients but also as a medium for host defense. Lysozymes and lactoferrin in human milk are thought to contribute to the development of intestinal microbiota beneficial to host health, especially for infants. However, lysozyme is deficient in milk from ruminant animals, especially cows. According to Prieur (1986), cow milk contains 3,000 times less lysozyme (0.13 μg/mL) than that of human milk (400 μg/mL). One solution to increase lysozyme in cow milk is to clone the genes to express the human lysozyme in cow mammary glands. This bio-engineered milk could improve immunity and prevent infection in infants and therefore be beneficial to the health of infants.
Ruminants have generally c-type lysozyme. Until now, 10 lysozyme genes have been identified in ruminants. There are at least 6 stomach lysozyme genes and 4 non-stomach lysozyme genes (i.e., mammary gland, kidney, trachea, and intestinal) in cows. Compared to other mammals, the expression level of cow lysozyme in the mammary gland, kidney, trachea, and intestines is low and in contrast to that in the stomach. The lysozyme gene in cow mammary gland tissue is expressed at very low levels in mammary gland tissue (<0.001% of the mRNA abundance) (Irwin, 2004). As in the case of humans, baboons, and mares lysozyme in milk, the cow milk lysozyme is also c-type. Milk lysozyme has been used to cure endometritis and mastitis of dairy cows (Hermann et al., 1973; Jauregui-Adell, 1975; Wang and Kloer, 1984; Steinhoff et al., 1994). Addition of lysozyme into milk and other dairy products can improve the quality of the milk and extend the shelf life (Brundige et al., 2010).
Yak (Bos grunniens) is a ruminant that can efficiently utilize alpine zone pasture resources on the Tibetan plateau. The yak has a high commercial value and has become an important ruminant mainly used for meat and milk production (He and Li, 2004). Compared to dairy cattle, yak has adapted to a high altitude ecosystem. Recently a new yak stomach lysozyme gene has been reported by Jiang et al. (2010). Interestingly, the phylogeny result suggests that it be closely related to the cow milk lysozyme and its amino acid (AA) sequence shares many common properties with the cow milk lysozyme (Jiang et al., 2010). However, there has been no accomplished comprehensive study about characterization of the milk lysozyme gene from yak mammary gland tissue. Therefore, we report the characterization of the yak milk lysozyme (YML) through the cloning of YML, the phylogenetic analysis, and its expression levels during the lactation cycle of yak mammary gland tissue and the lysozyme activities of the recombinant yak milk lysozyme (rYML) produced by Escherichia coli and Pichia pastoris.
All of the yaks in this research were obtained from the northwest plateau of Sichuan province in China. Mammary tissue samples (approximately, 1 g) from 3 lactating yaks were collected by biopsy of the right or left rear quarters at −30 (−27±3), −15, 1, 15, 30, 60, 120, 180, and 240 days relative to parturition (d). After skin incision, blunt dissection of the mammary capsule was performed to obtain mammary parenchyma. The incision sites were sutured and sprayed with topical antiseptic (10% Povidone Iodine Ointment, Taro Pharmaceutical Industries, Haifa Bay, Israel).
Tissue samples were rinsed in DEPC-treated water and then put into liquid nitrogen for further analysis. E. coli DH5α, BL21 (Novagen, Darmstadt, Germany) and P. pastors X33 (Invitrogen, Carlsbad, CA, USA) were used as the host strains for plasmid amplification and heterologous YML production. All experimental animal use in this study was approved by the institutional animal care committee of Southwest University for Nationalities followed the current guidelines on animal care (permit number: 2013-4-2).
Total RNA was extracted from mammary gland tissue using Trizol Reagent (Invitrogen, Grand Island, NY, USA). The extracted RNA was dissolved in DEPC water, and then stored at −80°C for further analysis. The purity and concentration of RNA samples were determined by an ultraviolet-visible spectroscopy (UV/Vis) spectrophotometer (Eppendorf, Hamburg, Germany).
The cDNA of each sample was prepared using a PrimeScript RT reagent kit with genomic DNA (gDNA) Eraser (Takara, Dalian, China) with 0.5 μg total RNA according to the manufacturer’s instructions. The contaminated gDNA was removed as follows: 2 μL 5×gDNA Eraser Buffer, 1 μL gDNA Eraser, 1 μL (0.5 μg) total RNA, 6 μL RNase free water were incubated at 42°C for 2 min and then cooled on ice for 2 min; and then, 4 μL 5×PrimeScript buffer, 1 μL PrimeScript RT Enzyme MixI, 1 μL RT Primer Mix, and 4 μL RNase free water were added to the previous mixture. Finally, the mixture was incubated at 37°C for 15 min and 85°C for 5 s for enzyme denaturation.
Based on the sequence of cattle milk lysozyme (http://www.ncbi.nlm.nih.gov/, GenBank No. JX946731), a specific primer set was designed to amplify the YML cDNA sequence (forward primer: 5′-ATGAAGGCTCTCCTTATTGTG-3′ and reverse primer: 5′-TTACACTCTGCAACCCTGAAC-3′) by Beacon Designer7.6 (http://www.premierbiosoft.com/). The polymerase chain reaction (PCR) reaction composition was as follows: 2.5 μL 10×Taq buffer, 2 μL 2.5mM Mg2+ solution, 1 μL dNTP Mix, 1 μL forward primer, 1 μL reverse primer, 1 μL template, 0.2 μL Ex Taq polymerase, and 16.3 μL DNase and RNase-free water in a total volume of 25 μL. PCR was performed according to the following program: at 94°C for 3 min for initial denaturation, and followed by 30 cycles of at 94°C for 30 s, at 58°C for 45 s, and at 72°C for 1 min for amplification and at 72°C for 10 min for final extension. The PCR fragment was recovered and ligated into pMD19-Tvector (Takara, China) to form a recombinant pMD19-T-YML, and then sequenced by 3730 DNA analyzer (ABI, Foster, CA, USA).
To express the YML gene in E. coli, four primer sets (i.e., YML1, YML2, YML3, and YML4; Table 1) were designed to include restriction enzyme sites during amplification of the YML gene. The reaction system and program parameters were same as the PCR to amplify the YML gene. The PCR products were purified and stored under −20°C.
To construct the recombinant expression vector, amplified PCR fragment (YML1, 462 bp; YML2, 411 bp) and the pET-32a expression vector (Novagen, Germany) were digested with BamHI and NdeI restriction enzymes. The other two PCR products (YML3 480 bp; YML4, 426 bp) and pET-32a expression vector were digested with BamHI and KpnI. After digestion, the PCR fragments were ligated into pET-32a vector using T4 DNA ligase (Takara, China) to form recombinant vectors, namely, pET32-YML1 pET32-YML2, pET32-YML3, and pET32-YML4 (Table 1). These recombinant vectors were transformed into E. coli BL21 cells (Novagen, Germany) and sequenced by 3730 DNA analyzer (ABI, USA) to confirm the insertion of the coding sequence of yak milk lysozyme. The inserted clones were incubated in LB medium included 50 μg/mL ampicillin to reach 0.5 in OD600 and then induced with 1 mM isopropyl beta-D-thiogalactopyranoside (IPTG) at 28°C for YML protein expression. After induction for 6 hours, 1 mL of the culture was removed and centrifuged at 5,000 rpm for 5 min. The pellet was suspended in 100 μL of DNase and RNase-free water and 100 μL of 2×loading buffer were added for sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). The SDS-PAGE was performed with 5% (vol/vol) concentrating and 15% (vol/vol) separating gels and the proteins were visualized by Coomassie Brilliant Blue R-250 staining. The remaining culture was centrifuged at 5,000 rpm for 5 min. The pellet was suspended in 4 mL 10 mM NH4Ac, and destroyed using the ultrasonic disrupter for 10 min with repetition of 6 s sonication and 9 s intervals under 500 W, and centrifuged at 12,000 rpm for 10 min. The supernatant was collected for lysozyme activity detection, and quantification of proteins was determined by Lowry method.
To express the YML gene in P. pastoris, a recombinant expression vector, that is, pPICZαA (Invitrogen, USA)-YML5 were constructed. At first, a primer set was designed to amplify the YML (i.e., YML5) (Table 1). The PCR condition was the same as we mentioned before. The PCR product was sequenced by 3730 DNA analyzer (ABI, USA) and then digested with restriction enzymes XhoI and XbaI. The PCR product was ligated into pPICZαA (Invitrogen, USA) that was digested with the same enzymes. The recombinant plasmid pPICZαA-YML5 (5 to 10 μg) was linearized with SacI and transformed into P. pastoris X33 by electroporation system (Bio-Rad, Hercules, CA, USA) and then screened on yeast extract peptone dextrose (YPD) plates containing 100 μg/mL Zeocin to select Zeocin-resistant recombinant clones according to the EasySelect Pichia Expression Kit (Invitrogen, USA). Expression of YML in P. pastoris X33 (Invitrogen, USA) was performed according to the Multi-Copy Pichia Expression kit (Invitrogen, USA). The Selected clones were then inoculated into buffered methanol complex medium (BMMY) medium and induced at 30°C for 96 h by adding methanol every 24 h to a final concentration of 1% (vol/vol). To purify the heterologous protein, rYML5, the supernatant of culture medium was obtained by centrifugation at 12,000 rpm for 5 min and concentrated using a Vacuum-Concentrator (Telstar, Terrassa-Barcelona, Spain). The concentrated supernatant was pooled and dialyzed against a 10 mM CH3COONH4 (pH = 5.8) buffer and applied onto a CM-Sepharose FF column which was eluted (1 mL/min) with a linear gradient of 300 mMCH3COONH4 (pH = 8.0) buffer. The active fractions were pooled and concentrated as above and then further purified using Sephadex G-75 (Sigma-Aldrich, St. Louis, MO, USA) with a 0.2% CH3COOH buffer. Fractions containing YML activity were concentrated and then characterized by SDS-PAGE and assessed for lysozyme activities.
Lysozyme activity of rYMLs was determined by the lysozyme detection kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) and all measurements were done in triplicates. The assay was conducted as follows: 2mL Micrococus lysodeikticus suspension was applied to each sample, i.e., 200 μL prepared rYML as test, 200 μL standard lysozyme solution at known units (200 UM/mL) as standard, and 200 μL water as negative control. The absorbance of the reaction solution was recorded at 37°C after 5 seconds (UT0), and two minutes 5 seconds later the data UT1 was recorded. The absorbance of a standard solution was also measured at the same time (ST0 and ST1). Lysozyme activity was calculated with the following formula:
Results were shown as specific activity units of lysozyme activity per milligram of purified rYML protein. The Oxford-cup test was performed to detect the antimicrobial activity of rYML5 with staphylococcus aureus (S. aureus) as the substrate. S. aureus was grown until a stationary phase and spread on 1% agar. Three Oxford plates were put onto the 90 mm plates. One was used for a negative control and the other two plates were for different amounts of recombinant YML protein. Purified YML protein was applied onto the Oxford plates and the agar plates were incubated at 37°C for 48 hours.
The expression level of the YML gene during the entire lactation cycle was analyzed using real time quantitative PCR (qPCR). Total RNA of mammary gland tissue were extracted and then the cDNA of the YML gene were synthesized by the method as described in the previous section. The primer set (forward primer: 5′-AACTACAATGCTGGAGAC-3′ and reverse primer: 5′-GGTAAATGACAGGCGTTA-3′) were designed by Beacon Designer 7.6 (http://www.premierbiosoft.com/) and the reference sequence was obtained from sequenced result of the YML gene. Three internal control genes (ICGs) for yak mammary gland were used in this experiment. The YML gene and 3 ICGs were run qPCR in triplicates by CFX96 Real-Time System (Bio-Rad, USA) using 2 μL cDNA from each time points, 5 μL Sso Fast EvaGreen supermix (Bio-Rad, USA), 0.5 μL each of 10 μM forward and reverse primers, 2 μL DNase and RNase-free water. The program set at 95°C for 5.0 min for initial denaturation, 39 cycles at 95°C for 10s, 55.7°C for 20 s for amplification, 95°C for 15 s plus 65°C to 95°C for 15 s for melting curve. A negative control without cDNA template was conducted in each assay.
The relative quantity for YML expression was obtained by CFX96 Real-Time System Manager software (Bio-Rad, USA) and presented as n-fold change relative to −30 d. The relative quantity for the YML gene was normalized by dividing using geometric mean of relative quantity data of selected ICGs. This final dataset was analyzed using a MIXED model with repeated measures in SAS 8.0 (SAS Institute Inc., Cary, NC, USA) to estimate the effect of time relative to parturition on gene expression. Compound symmetry was used for the data analysis. The model included the fixed effect of time (−30, −15, 1, 15, 30, 60, 120, 180, and 240 d) and the random effect of yak cow (Bionaz and Loor, 2008).
The sequenced cDNA fragment was searched against the non-redundant (nr) database at the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/, USA) using the Blast algorithm. Function-point, conserved domain, and signal peptide analysis were conducted by the online tool of motifs-PROSITE (http://cn.expasy.org/tools/scanprosite/), the Pfam database (http://pfam.sanger.ac.uk/), and the SignalP 3.0 Server (http://www.cbs.dtu.dk/services/SignalP/), respectively. The secondary structure of YML was predicted by online service (http://swissmodel.expasy.org/) and DNAMAN software (http://www.lynnon.com/).
To clarify the relationships among yak and cow lysozymes, AA sequences of YML (GenBank No. JX946731), yak stomach lysozyme (GenBank No. EU780011), Bos taurus milk lysozyme (GenBank No. AY684064), and other lysozymes retrieved from GenBank were aligned, and then the phylogenetic lysozyme relationships were determined with Mega version 5.0 with maximum likelihood (ML) and neighbor-joining (NJ) approach using Tamura-Nei evolutionary model (Tamura et al., 2011). The first ML tree was based on pig lysozyme sequence alignment with default settings. Bootstrap analysis with 1,000 replicas was used. The percentage of bootstrap replicas supporting each branch in the consensus tree was indicated above or to the right of the branch. Neighbor joining trees were shown with approximate branch lengths with the bar indicating 5% divergence. All of AA sequences of cow and yak lysozyme were obtained from the GenBank database.
The cDNA encoding the YML gene was amplified by qPCR. The desired PCR product (~447 bp) was obtained and encoded a 148 AA protein that contained a signal peptide of 18 AA followed by a mature 130 AA protein (Figure 1). The subsequent sequencing and analysis demonstrated that the cloned gene was the YML gene and then the sequence was submitted to the GenBank database (GenBank No.JX946731). The nucleic acid sequence identities between lysozyme of yak milk and one of cow milk and between lysozyme of the yak milk and yak stomach were 89.04% and 84.60%, respectively, and the identities of corresponding AA sequence were 80.41% and 69.59%, respectively. Sequence analysis of the YML gene showed that the molecular mass of the mature protein was 14.66 kDa and the estimated isoelectric point was 7.99. Further sequence analysis showed that the YML protein contained 8 cysteine residues (position at 24, 48, 83, 95, 99, 113, 134, and 146; Figure 1). It also belonged to the c-type lysozyme that contained 53 Glu (E) and 71 Asp (D) as its active site included other known milk lysozymes in cow, sheep, and goats. It contained 7 α-helix (position at 1 to 13, 19 to 32, 35 to 52, 101 to 102, 107 to 118, 123 to 128, 130 to 131) and 3β-sheet (position at 60 to 61, 74 to 76, 80 to 82) which were same to other milk lysozymes (Figure 1).
The YML gene was heterologously expressed using pET-32a/E. coli system. Four YML cDNA fragments with different expression tags (YML1, YML2, YML3, and YML4) were produced and ligated into pET32a vector and then transformed into E. coli to form recombinant clones (Table 1 and Figure 2). Four heterologously expressed rYML proteins were obtained from the selected recombinant clones. Molecular masses of rYMLs were rYML1 (16.54 kDa), rYML2 (14.66 kDa), rYML3 (33.45 kDa), rYML4 (31.69 kDa) which were detected by SDS-PAGE (Figure 3a–3d). The large size of the fusion protein is due to the leading peptide (16.91 kDa) encoded by the expression vector. Lysozyme activities of crude extract of recombinant clones were as following: rYML1 (M = 921.00 U/mg, SD = 1.63), rYML2 (M = 512.60 U/mg, SD = 1.81), rYML3 (M = 412.00U/mg, SD = 7.46) and rYML4 (M = 106.00 U/mg, SD = 5.87).
rYMLs was also expressed by selected recombinant P. pastoris clones. The heterologously expressed rYML5 was purified by CM-Sepharose FF and Sephadex G-75 column (Figure3e). Lysozyme activity of the purified rYML5 was 1,864.24 U/mg (SD = 25.75). The agarose diffusion assay showed that addition of 96 μg rYML5 into the well clearly inhibited the growth of S. aureus (Figure 4), whereas the negative control did not. The antibacterial activity of YML might be attributed to its capacity to disrupt bacterial murein. This indicated that the milk lysozyme still can be used as an antibacterial peptide although ruminant milk have lysozyme deficiency.
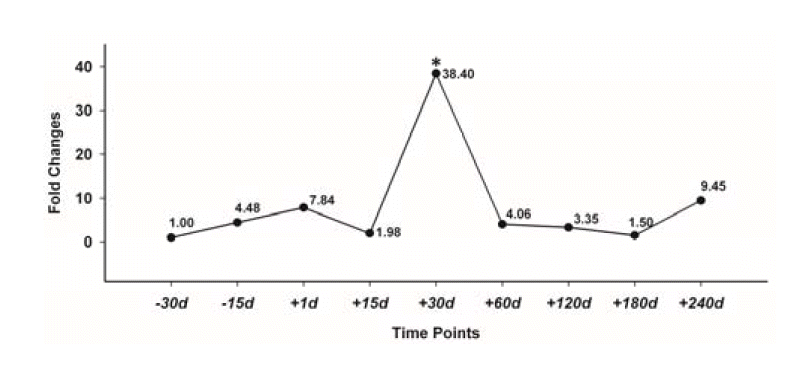
Overall, the expression level of the YML gene was higher during lactation than dry period (Figure 5). Expression of the YML gene was significantly increased during lactation and up regulated to ~38 fold at 30 d (p<0.01). Then the expression was significantly decreased from time point 30 d to 180 d with a tiny increase at the end of lactation. The fold changes of these time points varied from 2 to 10 fold compare to −30 d before parturition but no significant differences were found among time point −30, −15, 1, 15, 60, 120, 180, and 240 d. Interestingly, the statistical results indicated that significant differences could be found between 30 d vs −30 d and the remaining time points (i.e., statistical effect p<0.05 for all point against 30 d).
Alignment of the AA sequence shows that the sequence identity between YML (GenBank No.JX946731) and cow lysozyme (GenBank No. HQ285242), YML and cow milk lysozyme (GenBank No. AY684064), YML and yak stomach lysozyme (GenBank No. EU780011) are 95.27%, 80.41%, and 69.59%, respectively (Figure 6). The phylogenetic tree indicated that the YML was diverged from the stomach lysozyme before the recruitment of lysozyme as a digestive enzyme. Interestingly, when the tree was constructed with a combination of cow and yak lysozymes, the YML and cow kidney lysozyme were tightly grouped first and then grouped with cow milk lysozyme and a published yak stomach lysozyme gene. Cow stomach lysozymes were tightly grouped, and then grouped with two unpublished yak stomach lysozymes, and cow tracheal and intestinal lysozymes.
Despite the lack of a lysozyme in milk (Prieur, 1986), ruminant animals have more lysozyme genes, i.e., 10 genes were found in cow genome until now, expressed in non-stomach tissue including tracheal, mammary glands, kidney and intestine tissues than other mammals (Gallagher et al., 1993). But in the case of their stomach tissue, the expression level of lysozyme is extremely high and the antibacterial lysozymes are recruited as digestive enzymes which allow ruminant animals to exploit plant materials as food resources. Lysozymes in mammals typically have antibacterial functions for host defense. Lysozyme in non-stomach tissue of ruminants shows that it plays a role as found in other mammals. Interestingly, our previous study showed that the sequence properties of a yak stomach lysozyme were more similar to the cow milk lysozyme (Jiang et al., 2010). To clarify the relationship between yak stomach and milk lysozymes, YML was cloned and analyzed in this study.
In our study, the YML gene from yak mammary gland tissue was cloned. Through sequence analysis and lysozyme activity test of rYMLs, we found that cloned the YML gene is a new c-type lysozyme gene. Similar to lysozymes from other ruminants, the YML contains 148 AA including a signal peptide of 18 AA residues. Even though the AA residues of ruminant lysozymes might be various (position at 143 to 148), all of them contain a signal peptide of 18 residues and share common cleavage site at the conserved VQG-K in its N-terminal regions (Figure 1) and are secreted protein. AAs sequence alignment of milk lysozymes showed the similarity between yaks and cattle, sheep and goats was 80.41%, 74.32%, and 73.65%, respectively. The estimated isoelectric point (pI) of the milk lysozyme of yak, cattle, sheep, and goats was 7.99, 10.34, 9.97, and 9.97, respectively. The estimated pI of a previously cloned yak stomach lysozyme was 10.64. This means that the YML is more closely related to the milk lysozyme of cattle but their protein properties are different. The protein structure shows that the YML has the same secondary structure as other ruminant milk lysozymes. It also contains the same active sites (53 Glu, E; 71 Asp, D) as other c-type lysozymes. This implies that the YML also has antibacterial function in mammary gland for host defense.
To select the best method to express the YML in pET/E. coli system, the YML was heterologously expressed in E. coli and P. Pastoris. Four kinds of rYMLs were produced in E. coli by adding different expression tags. It was found that the enzyme activity of rYML1 (non-fusion with native signal peptide of the YML) was the highest compared to those of other constructs rYMLs (rYML2, rYML3, and rYML4) expressed in E. coli. The lowest one was rYML4 which was labeled with a Tag at position of Trx, His, and S and N-terminus of the mature peptide of YML. It seems that the YML expressed only as native signal peptide without an expression tag from pET32 vector can improve the enzyme activity when the heterologous YML was expressed in E. coli. Recombinant lysozyme expressed in E. coli was reported in several papers (Zhang et al., 2014). There are 8 cysteines in YML which form four disulfide bonds. The renaturation of disulfide-containing lysozyme was highly affected by the extent of denaturation of YML in E. coli. This may be the reason of the lower enzyme activity of rYML1 to 4 compared to that of rYML5 (Lin et al., 2007).
Contrasted to E. coli as expression system, the highest enzyme activity was detected in heterologously expressed rYML5 (1,864.24 U/mg) in P. Pastoris. Some researchers also reported that recombinant lysozyme is expressed in methylotrophic yeast (Wang et al., 2011). Recombinant lysozyme expressed in methylotrophic yeast showed significant activity against S. aureus. The main advantage of yeasts as expression systems is that yeasts can provide better protein folding pathways for eukaryotic proteins, and glycosylation of proteins (Cereghino and Cregg, 2000). Our result shows that yeasts can be used as a potential system to produce YML. The highest lysozyme activity of rYML5 expressed in P. pastoris verifies that this yeast system is a better than E. coli to produce the YML.
Some research demonstrated that very low mRNA level of milk lysozyme (<0.001% of the mRNA abundance) in mammary glands might be due to the low protein yields encountered in the protein purification process (White et al., 1988). It can be deduced that a similar expression pattern of YML may occur in the yak’s mammary glands. In current study, the expression level of the YML mRNA was assessed during the whole lactation cycle. In Figure 5, we illustrate that the YML gene was expressed at a higher level during lactation period than non-lactation period. The highest expression peak appeared at time point 30 d postpartum. This implies that lysozyme concentration of yak milk might be comparatively high in “early” lactation stage which may confer passive protection for neonates against pathogens.
Previous evolutionary studies of lysozyme genes in ruminants revealed that there are three groups of lysozyme genes in cows, specifically: i) stomach lysozymes, ii) a kidney lysozymes, and iii) other non-stomach lysozymes (Irwin, 2004). To study the lysozymes of yak, our group has already cloned three yak stomach lysozyme genes. Interestingly, the sequence of one stomach lysozyme gene was similar to cow’s milk lysozyme (Jiang et al., 2010). Further analysis, revealed that this lysozyme possessed a few of the adaptive changes necessary for the ruminant stomach lysozyme which always shifts its pH optimum and evolves its AA sequence to increase acid and pepsin resistance. So we can speculate the reason that yak stomach lysozyme is different from normal stomach lysozyme of ruminant animals and whether yak stomach lysozyme is the intermediate of stomach lysozyme and milk lysozyme. Since the pI value of pseudogene from stomach, PsiNS4, also varies between 7.5 and 10.2, and the pI value in the case of yak stomach lysozyme was 10.58, it was necessary to determine whether the cloned stomach lysozyme was a pseudogene. Previously, our results showed yak stomach lysozyme was not a pseudogene because it also had lysozyme activity when its recombinant protein was expressed in E. coli (Jiang et al., 2010). To clarify this problem, the YML gene was cloned in this study. Our phylogeny analysis with AAs of yak lysozyme suggested that there are three groups of lysozymes in yaks. The results indicated that two yak stomach lysozymes were closely related to a previously reported yak stomach lysozyme (GenBank No. EU780011). The YML cloned in this study was not related to yak stomach lysozyme. It indicated that it was derived from a different gene other than a stomach lysozyme gene, that is, cow milk lysozyme (Steinhoff et al., 1994). Since the remaining lysozymes were not cloned in yak, a phylogenetic analysis including all cow and yak lysozymes was conducted. The results showed that the YML was more closely related to the cow kidney lysozyme and the previously reported yak stomach lysozyme was closely related to cow milk and the cow ΨNS4 pseudogene. As mentioned by Irwin (2004), cow stomach lysozymes formed a tight group with one another. Cow tracheal lysozyme, intestinal lysozyme, and two yak lysozymes were strongly related and then grouped with cow stomach lysozymes. The phylogenetic tree also showed that two yak stomach lysozymes were closely related to cow tracheal, intestinal, and stomach lysozymes. Each yak stomach lysozyme grouped with cow milk, ΨNS4, and the YML was closely related to the cow kidney lysozyme. This reflects the hypothesis that yak lysozymes were evolved in the same manner as cow lysozymes, but the duplication and recombination was different from that in cows. The similarity in evolution was also found in the goat tears lysozyme. Sequence analysis of the goat tears lysozyme indicated that they might be diverged from the stomach lysozyme family by an ancient duplication and later duplications are probably responsible for the multiple forms of tear and milk lysozymes in goats (Jollès et al., 1990). Interestingly, YML and cow kidney lysozyme formed a closely related group. The cow kidney lysozyme was always regarded as an individual group but the cow milk lysozyme seems to have been derived from a different gene other than the cow stomach lysozyme gene (Steinhoff et al., 1994). This implies that the YML has a different ancestor gene similar to its counterpart in cow mammary glands.
In our research, we cloned a new c-type lysozyme from yak mammary gland. Phylogenetic analysis of YML indicated that it may have been derived from a different ancestor gene than its counterpart in cows. The YML gene is markedly up regulated at 30 day parturition indicated that it plays a role in early milk. Heterologous expression of the YML showed that P. pastoriswas a better system to produce rYML than E. coli. Finally, we issued the base of longitudinal evolutionary study of the yak lysozyme through our results and recommend that newly cloned c-type milk lysozyme from yak can be utilized as an antibacterial product in human food and the additives for domestic animals.
ACKNOWLEDGMENTS
This work was supported by a grant from The National Natural Science Foundation of China (31172198), Sichuan Youth Science and Technology Fund (09ZQ026-011), and The Construction Project of Postgraduate Academic Degree in Southwest University for Nationalities (2011XWD-S071007).
Figure 1
Alignment of amino acids of yak, cattle, sheep, goat milk lysozymes, and yak stomach lysozymes. The signal peptide is in bold and italics; the active sites are in bold and boxed; cysteine residues are in bold and shaded; Asp-Pro bond is bold. The secondary structural elements are listed under the sequence (7 α-helixes, 3 -sheets and 10 random coils). Accessed GenBank No.: JX946731 in yak, AY684064 in cattle, XP_004022875 in sheep, XP_005680291 in goat milk lysozymes and EU780011 in yak stomach lysozyme.

Figure 2
Polymerase chain reaction product of yak milk lysozyme (YML) gene amplified by specific primer sets. The size of each fragment is marked under their bands. M1, 250bp ladder; M2, DL2000 ladder; M3, DL2000; M4, λ-EcoT14. Lane1, YML1; Lane 2, YML4; Lane 3, YML2; Lane 4, YML3; Lane 5, YML5.

Figure 3
SDS-PAGE analysis of heterologously expressed yak milk lysozyme (YML) in E. coli and P. pastoris. (a) Recombinant yak milk lysozyme1 (rYML1) expressed in E. coli M, protein maker; 1, pET32a after induction for 6 h; 2, pET32a+YML1after induction for 6 h. (b) rYML2 expressed in E. coli M, protein maker; 3, pET32a after induction for 6 h; 4, pET32a+YML2 after induction at 0 h, 5, pET32a+YML2 after induction for 6 h. (c) rYML3 expressed in E. coli M, protein maker; 6, pET32a after induction for 6 h; 7, pET32a+YML3 after induction for 2 h; 8, pET32a+YML3after induction for 6 h. (d) rYML4 expressed in E. coli M, protein maker; 9, pET32a after induction for 6 h; 10, pET32a+YML4 after induction for 2 h; 11, PET32a+YML4 after induction for 6 h. (e) rYML5 expressed in P. pastoris. M, protein Marker; 12, cultured supernatant of the recombinant P. pastoris; 13, purified rYML5. SDS-PAGE, sodium dodecyl sulfate polyacrylamide gel electrophoresis.

Figure 4
Antimicrobial activities of the purified recombinant yak milk lysozyme5 (rYML5) against Staphylococcus aureus. The information of plate number is as following: a negative control (0 μg, Plate 1), purified rYML5 (48 μg, Plate 2), purified rYML5 (96 μg, Plate 3).
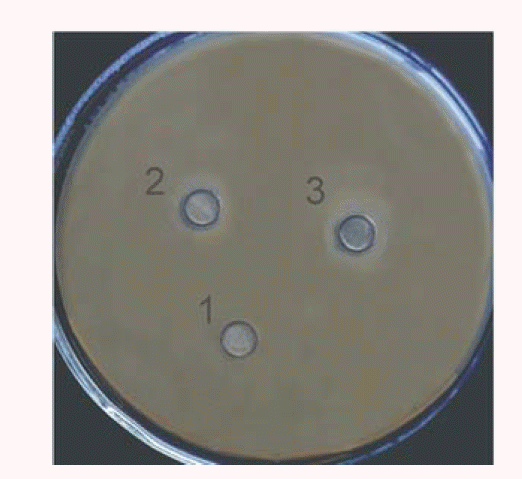
Figure 5
Expression pattern of the yak milk lysozyme (YML) gene during the lactation cycle. Each value indicates the least squares means of each time point and gene expressed level is calculated as n-fold change relative to −30 d at late pregnancy. Pooled standard error of the mean of YML is 8.26. * Statistically different from −30 d (p<0.01).
REFERENCES
Bionaz M, Loor JJ. 2008. Acsl1, agpat6, fabp3, lpin1, and slc27a6 are the most abundant isoforms in bovine mammary tissue and their expression is affected by stage of lactation. J Nutr 138:1019–1024.


Brundige DR, Maga EA, Klasing KC, Murray JD. 2010. Consumption of pasteurized human lysozyme transgenic goats’ milk alters serum metabolite profile in young pigs. Transgenic Res 19:563–574.



Cereghino JL, Cregg JM. 2000. Heterologous protein expression in the methylotrophic yeast pichia pastoris. FEMS Microbiol Rev 24:45–66.


Corin K, Pick H, Baaske P, Cook BL, Duhr S, Wienken CJ, Braun D, Vogel H, Zhang S. 2012. Insertion of t4-lysozyme (t4l) can be a useful tool for studying olfactory-related gpcrs. Mol Biosyst 8:1750–1759.


Gallagher DS, Threadgill DW, Ryan AM, Womack JE, Irwin DM. 1993. Physical mapping of the lysozyme gene family in cattle. Mamm Genome 4:368–373.


Hannig C, Spitzmüller B, Hoth-Hannig W, Hannig M. 2011. Targeted immobilisation of lysozyme in the enamel pellicle from different solutions. Clin Oral Investig 15:65–73.


He AX, Li L. 2004. Study on the relation between yak performance and ecological protection. In : Proceedings of the Fourth International Congress on Yak; September 20–26; Chengdu, China.
Hermann J, Jollès J, Buss DH, Jollès P. 1973. Amino acid sequence of lysozyme from baboon milk. J Mol Biol 79:587–595.


Jauregui-Adell J. 1975. Heat stability and reactivation of mare milk lysozyme. J Dairy Sci 58:835–838.


Jiang M, Chen Y, Wang Y, Loor JJ, Ye Y, Wen Y, Zi X, Cai Y, Drackley JK. 2010. Yak (bos grunniens) stomach lysozyme: Molecular cloning, expression and its antibacterial activities. Anim Biotechnol 21:25–35.


Jollès J, Prager EM, Alnemri ES, Jollès P, Ibrahimi IM, Wilson AC. 1990. Amino acid sequences of stomach and nonstomach lysozymes of ruminants. J Mol Evol 30:370–382.


Kastorna A, Trusova V, Gorbenko G, Kinnunen P. 2012. Membrane effects of lysozyme amyloid fibrils. Chem Phys Lipids 165:331–337.


Lan F, Hu H, Jiang W, Liu K, Zeng X, Wu Y, Gu Z. 2012. Synthesis of superparamagnetic fe3o4/pmma/sio2 nanorattles with periodic mesoporous shell for lysozyme adsorption. Nanoscale 4:2264–2267.


Lin JL, Ruaan RC, Hsieh HJ. 2007. Refolding of partially and fully denatured lysozymes. Biotechnol Lett 29:723–729.


Marsh MB, Rice CD. 2010. Development, characterization, and technical applications of a fish lysozyme-specific monoclonal antibody (mab m24-2). Comp Immunol Microbiol Infect Dis 33:e15–23.



Ng A, Heynen M, Luensmann D, Jones L. 2012. Impact of tear film components on lysozyme deposition to contact lenses. Optom Vis Sci 89:392–400.


Prieur DJ. 1986. Tissue specific deficiency of lysozyme in ruminants. Comp Biochem Physiol B 85:349–353.


Proctor VA, Cunningham FE. 1988. The chemistry of lysozyme and its use as a food preservative and a pharmaceutical. Crit Rev Food Sci Nutr 26:359–395.


Steinhoff UM, Senft B, Seyfert HM. 1994. Lysozyme-encoding bovine cdnas from neutrophile granulocytes and mammary gland are derived from a different gene than stomach lysozymes. Gene 143:271–276.


Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. Mega5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739.



Wang CS, Kloer HU. 1984. Purification of human lysozyme from milk and pancreatic juice. Anal Biochem 139:224–227.


Wang N, Wang Y, Li G, Sun N, Liu D. 2011. Expression, characterization, and antimicrobial ability of t4 lysozyme from methylotrophic yeast hansenula polymorpha a16. Sci China Life Sci 54:520–526.


White FH, McKenzie HA, Shaw DC, Pearce RJ. 1988. Studies on a partially purified bovine milk lysozyme. Biochem Int 16:521–528.

- TOOLS






 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print





