Effects of Chromium Methionine Supplementation on Blood Metabolites and Fatty Acid Profile of Beef during Late Fattening Period in Holstein Steers
Article information
Abstract
The objective of this study was to determine the effects of chromium methionine (Cr-Met) chelate supplementation on blood metabolites and fatty acid profile of beef from Holstein steers during late fattening period. Fifteen Holstein steers were allotted randomly into two groups including the control (non Cr-Met feeding, NCM, ave. body weight [BW] = 483±25.7 kg) and the treatment (Cr-Met feeding for 4 months, 4CM, ave. BW = 486±27.5 kg) group. The feeding amount of Cr-Met to animals was limited to 400 ppb/cow/d and was supplemented to total mixed ration. No difference in blood albumin, alkaline phosphatase, urea-nitrogen, calcium, creatine, glucose, total protein, triglyceride, and cholesterol were observed between the treatment groups (p>0.05). The level of high density lipoprotein was higher in the 4CM group than the NCM group, whereas low density lipoprotein was lower in the 4CM group (p<0.05). The fatty acid composition (caprate, laurate, myristate, pentadecanoate, palmitate, palmitoleate, margarate, cis-11 heptadodecanoate, stearate, oleate, trans-vaccenate, linoleate, cis-11 eicosenoate, docosa hexaenoic acid, and docosa pentaenoic acid) of the beef showed no difference between the two groups (p>0.05). The arachidonic acid level tended to be higher in the 4CM than the NCM group (p = 0.07). Cr-Met had no influence (p>0.05) on the ratio of saturated, unsaturated, unsaturated/saturated, monounsaturated/saturated and polyunsaturated/saturated fatty acids whereas the ratio of polyunsaturated fatty acids (PUFA) in the 4CM group was comparatively higher than the NCM group (p<0.05). This study concluded that feeding Cr-Met supplementation in 400 ppb/d to Holstein steers for 4 months during late fattening period can improve some blood metabolites and beef quality by increasing PUFA and gamma-linoleate compositions of beef.
INTRODUCTION
A recent study has reported that Cr-Met in the form of chelate could improve carcass characteristics including marbling score in Korean native steers (Sung et al., 2015). Another follow up study in Holstein steers during raising and late fattening period showed that 4 months is an optimum period for feeding Cr-Met chelate to improve daily gain and carcass characteristics of Holstein steers during the fattening period; however, fatty acids profile of beef was not measured (Song et al., 2013). Therefore, the apparent effects of Cr-Met chelate supplementation on blood metabolites and profile of fatty acids of beef in Holstein beef steers during late fattening period has not yet been investigated.
Chromium (Cr) is one of the essential micronutrients for ruminants and is considered to be a metabolic modifier. The organic source of Cr is a promising form due to higher bioavailability than inorganic sources (NRC, 1997). Cr supplementation has been reported to show an influence on some blood metabolites such as glucose (Wang et al., 2007) and could improve carcass quality such as intermuscular fat and percentage of muscle (Boleman et al., 1995) in pigs. Furthermore, Cr is also known to be a key constituent of glucose tolerance factor (regulates blood glucose level) and maintains glucose homeostasis (Sung et al., 2015). In addition, supplemental Cr was also found to play an important role in serum cholesterol homeostasis (Ohh et al., 2004). Cr supplementation decreased the level of total cholesterol, low density lipoprotein (LDL) cholesterol, and triglyceride in the blood, but increased the level of high density lipoprotein (HDL) cholesterol (Anderson, 1995; Sung et al., 2015). Schwarz and Merts (1959) and Bunting et al. (1994) reported that Cr supplementation can alter glucose metabolism in rats and in calves. Positive responses were reported in pigs by Page et al. (1993), Lindemann et al. (1995), Boleman et al. (1995), and Mooney and Cromwell (1995, 1997) in swine for carcass leanness. Moreover, according to Ohh et al. (2004), the positive effect of Cr supplementation can be associated with its obvious influence on the systematic division of energy between adipose and lean tissue. Reports of lessened fat over the 10th rib and decreased yield grades have been reported in lambs supplemented with Cr tripicolinate (Kitchalong et al., 1995). However, others reported no responses in carcass leanness to supplemental Cr (Harris et al., 1995; Ward et al., 1995). Likewise, Cr research has been conducted in dairy (Al-Saiady et al., 2004) and beef cattle (Swanson et al., 2000; Pollard et al., 2002; Stahlhut et al., 2006). Treatments were control with no Cr-Met supplementation and the different level of Cr-Met supplementation in diet with or without yeast resulted in improving carcass quality including marbling score and glucose tolerance rate. The aim of the present study was to evaluate the effects of Cr-Met chelate on blood metabolites and fatty acids profile of beef from Holstein beef steers.
MATERIALS AND METHODS
Treatments, feeding and experimental procedure
To assess the effect of Cr-Met on performance and beef quality, fifteen Holstein steers were randomly assigned into two dietary treatment groups; Non Cr-Met feeding (NCM, 7 head), Cr-Met feeding over a 4 months (4CM, 8 head). Average body weights of groups were 483±25.7 kg, 486±27.5 kg for NCM and 4CM, respectively at the beginning of the experiment. The feeding amount of Cr-Met (Innobio Co., Ltd., Shiheung, Korea) to animals was limited to 400 ppb/cow/d. The duration for the study was 4 months. The rate of forage to concentrate was 20:80 and forages included alfalfa cube and perennial ryegrass hay according to NRC requirements of beef cattle (2000). Concentrate, alfalfa cubes and Perennial ryegrass hay were fed twice daily, in the morning at 0900 am and at 0500 pm in the afternoon to achieve controlled feeding of fixed ratio of F:C as 20:80. The intake of concentrate was 8±0.4 kg (dry matter [DM]) and the forage intake was 2 kg (1±0.2 kg of alfalfa cube +1±0.2 kg of Perennial ryegrass hay, DM) for both groups. The composition of the experimental diets is presented in Table 1.
Feed, blood and beef quality analysis
Common nutrients were analyzed according to AOAC (1990); neutral detergent fiber and the level of acid detergent fiber were analyzed using the method of Goering and Van Soest (1991). A total digestible nutrient (Table 1) was calculated using the regression equation Wardeh (1981). Steers had ad libitum access to water. Blood samples from each steer at the beginning and the end of the experiment was collected from the jugular vein at 1300 h by using a vacutainer (no additive: BD, Franklin Lakes, NJ, USA) for serum separation. The serums samples were collected at the beginning, on a monthly basis and at the end of the experiment by centrifuging it at 2,500×g for 15 minutes and the samples were stored at 20°C for further processing. The serum samples were later analyzed for total protein, albumen, alkaline phosphatase (ALP), calcium, creatine, triglyceride, cholesterol, glucose, LDL, and HDL, using kits abiding with the manufacturer’s protocol (Modular analytic E170, Roche, Germany). Upon completion of the field experiment, all steers were slaughtered in order to measure the fatty acids composition of beef from loin side. Samples from each steer were frozen at 20°C for 12 hours, and were thawed prior to analysis. According to the method of lipid extraction (Folch et al., 1957), 6 g of sample and chloroform/methanol (2:1) solution were homogenized in a 25 mL homogenizer (Diax 6000, Heidolph, Germany) at 1,100×g for 30 seconds. Next 6 mL of 0.88% KCl solution was added to the homogenate, followed by centrifugation at 2,500×g (GS-6R Centrifuge, Beckman, Ramsey, MN, USA) for 10 minutes. The fluid was filtered through filter paper and lipid was concentrated using a nitrogen gas concentrator (MGS-2200, Eyelaa Tokyo Rikakikai Co., Ltd, Tokyo, Japan) following the method of AOAC (1990). Each of the fatty acid methyl ester standards (Sigma-Aldrich Co., Saint Louis, MO, USA) was qualitatively compared with retention time, and the analytic conditions used for Gas Chromatography (Agilent 6890N, Agilent Technologies, Santa Clara, CA, USA). For this, a sample of beef and tissue was kept for the split ratio of 1:10. The oven injector was heated with 220°C. A carrier of gas 1mL/min was heated with 150°C for one min. A column HP-Innowax (30 m length×0.32 id×0.25 μm thicknesses) maintained for a detector temperature of 275°C. The oven maintained the temperature of 200°C to 250°C at 3°C/min and 250°C for 5 min.
Statistical analysis
All the data are reported as the sample mean±the standard deviation. Pairwise comparisons between means of different groups were performed using a t-test. The difference between two subsets of data is considered statistically significant if the t-test gives a significance level p (p value) less than 0.05.
RESULTS AND DISCUSIONS
Blood metabolites
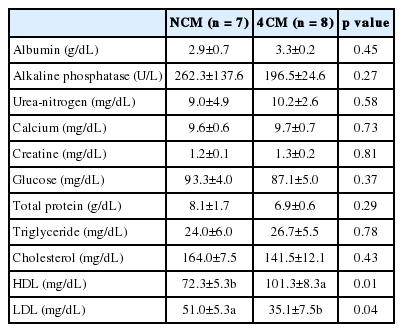
In assessing the effect of Cr-Met supplementation on normal body functions (renal function, clinical enzymology etc.) we observed that Cr-Met supplementation did not affect (p>0.05) albumen, ALP, calcium and creatine in blood (Table 2). Al-Saiady et al. (2004) also did not find any change in the albumin level in Holstein cows supplemented with chelated Cr. Similar results for creatine levels in pigs was observed by Lindemann et al. (2008). Kaneko et al. (1989) reported that an increase in ALP activity may occur in response to bone or liver damage. However, in the current study no increase (p>0.05) in ALP activity was observed. Furthermore, no effect on urea nitrogen and total protein was observed in any of the other trials (Ohh and Lee, 2005; Sung et al., 2015). Similar results were reported by Kitchalong et al. (1995) in lambs and Bunting et al. (1994) in steers with Cr-picolinate. However, Dominguez-vara (2009) showed inconsistent results in urea nitrogen levels with the addition of Cr-yeast. In this study, supplementation with Cr-Met chelate did not affect (p>0.05) serum glucose levels in Holstein steers. Previous studies (Kegley et al., 1997) also found no effect of Cr supplementation on plasma glucose concentration in cattle; however, reports by Chang et al. (1995) and Stahlhut et al. (2006) demonstrated that Cr supplementation lowered plasma glucose concentration in growing and finishing steers. Blood cholesterol has a diagnostic value for situations like hypothyroidism (Kaenko, 1989). However, no difference (p>0.05) was observed between triglyceride, and cholesterol in the current study (Table 2). Lindemann et al. (2008) also found no deviation in cholesterol and triglyceride values from that of the control group. Their findings support the results of this study. Bunting et al. (1994) reported decreased cholesterol levels in unstressed calves fed with Cr-picolonate. Supplemental Cr also plays an important role in serum cholesterol homeostasis. Cr supplementation decreased the level of the blood’s total cholesterol, LDL cholesterol, and triglyceride but increased the level of HDL cholesterol (Anderson, 1995). Dietary saturated fat and cholesterol lead to elevated levels of cholesterol in the blood. A possible mechanism of Cr on amino acid synthesis has been predicted due to the role of insulin in amino acid uptake. However, other activities of Cr in protein metabolism have not been reported (Roginski and Mertz, 1969). In this study, HDL was remarkably increased whereas LDL was decreased (p<0.05, Table 2). This result is in agreement with Sung et al. (2015) who reported higher HDL and lower LDL levels in Korean native steers fed with supplemented Cr.-Met chelate. Al-Saiady (2004), however, reported increased cholesterol levels in dairy cows with Cr supplementation. HDL helps to prevent narrowing of the artery walls by removing excess cholesterol and transporting it to the liver for excretion. LDL carries cholesterol for cell building needs, but leaves behind any excess on artery walls and in tissues (Wang et al., 2007; Sung et al., 2015). High LDL and low HDL levels indicate diets high in refined carbohydrates and/or carbohydrate sensitivity. Low levels of HDL are strong indicators of insulin resistance, but in this experiment we observed adverse amounts due to using Cr-Met in diets.
Fatty acid composition of beef
In the current study, no significant differences (p>0.05) in composition of fatty acids (caprate, laurate, myristate, pentadecanoate, palmitate, palmitoleate, margarate, cis-11 heptadodecanoate, stearate, oleate, trans-vaccenate, linoleate, cis-11 eicosenoate, docosa hexaenoic acid, and docosa pentaenoic acid) were observed. Whereas, gamma linolenate (C18:3n6, p<0.05) and arachidonic acid (C20:4n6, p = 0.07) were higher in the 4CM group than the NCM group. There was no difference (p>0.05) in gamma linolenate level between the two treatment groups. The level of arachidonic acid in 4CM was higher (p<0.05) than other fatty acids (Table 3). The fatty acid composition of beef plays an important role in the quality of beef for consumers (Sung et al., 2015). Cr supplementation has been employed to manipulate the quality of beef due to its biological function on body fat and muscle metabolism (Page et al., 1993; Sung et al., 2015). Plasma non-esterified fatty acids (NEFA) reflect body fat mobilization in response to a negative energy balance or stress conditions. During an energy deficit, animals break down triglycerides (fat) stored in adipose tissue. NEFA enters the blood stream to be transported to organs and tissue throughout the body. The concentration of NEFA measured in blood has been shown to reflect fat mobilized from body fat reserves. Plasma NEFA concentrations were lower in beef cows receiving supplemental Cr shortly after calving (days 97 and 155), especially young cows (Stahlhut et al., 2006). Matthews et al. (2001) reported that swine supplemented with Cr picolinate had lower plasma NEFA concentration vs. control. Similarly, studies on sheep (Kitchalong et al., 1995) and dairy cattle (Hayirli et al., 2001) found that Cr supplementation lessened plasma NEFA concentration. Conversely, supplemental Cr had no effect on plasma NEFA concentration in growing steers (Chang and Mowat, 1992; Bunting et al., 1994). In a study by Stahlhut et al. (2006), NEFA concentration responses to Cr were affected by the amount of copper. Cr supplementation decreased plasma NEFA concentration in non- copper supplemented cows. Beef quality could have been different at the beginning of the experiment but we were unable to slaughter the steers at both the beginning and the end of the experiment. However, the body weight of steers at the beginning of the experiment showed no significant difference while cows were receiving specific amounts of feed through controlled feeding. Therefore, any changes to the fatty acids profile of beef may be due to Cr-Met supplementation since the intake of DM was similar between the two groups and blood parameters were not different at the beginning of the experiment. The response to organic Cr supplementation in beef quality of farm animals is varied among animal species, form of dietary Cr, and the level of Cr supplementation. However, the results have varied presumably due to other extrinsic factors such as onset and level of supplementation, nutrients and level of basal diet, breed and species (Page et al., 1993).

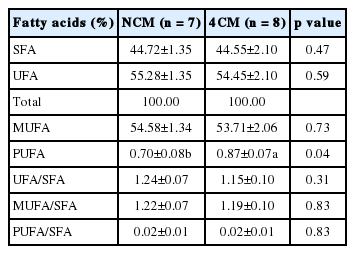
Effect of Cr-Met supplementation on fatty acid profile of beef (loin side) from Holstein steers during late fattening period
Cr-Met had no effect on the ratio of saturated, unsaturated, unsaturated/saturated, monounsaturated/saturated, and polyunsaturated/saturated fatty acids while the ratio of polyunsaturated fatty acids (in the 4CM group was considerably higher (p<0.05) than the control group (Table 4). Cr can affect fat mobilization from body stores to meet nutrient demands. In this regard, alteration in some fatty acid compositions can be explained. Cr in the form of chelate especially with methionine has a greater potential to be absorbed through increased digestion in the gastrointestinal tract (Mertz, 1993; Ohh et al., 2004; Sung et al., 2015). Since cattle tend to produce less intramuscular fat, it is expected that the polyunsaturated fatty acid (PUFA) level of the fat will be higher. In this study higher (p<0.05) PUFA in 4CM was observed. The finishing diet strongly influences the fatty acid composition of beef (Smith et al., 2009). Zea et al. (2007) reported that saturated fatty acids (SFA) were higher in animals fed with concentrates, while animals fed with silage had higher levels of PUFA and a higher PUFA/SFA ratio. Furthermore, Smith et al. (2009) reported that grain feeding arouses the activity of adipose tissue stearoyl-CoA desaturase in marbling adipose tissue and lowers ruminal isomerization/hydrogenation of dietary PUFA, resulting in a noticeable increase in monounsaturated fatty acid (MUFA) in beef over time. The current study found no differences (p>0.05) in the values of SFA, unsaturated fatty acid (UFA), MUFA, UFA/SFA, MUFA/SFA, and PUFA/SFA.
CONCLUSIONS
It can be concluded that feeding Cr-Met supplementation in 400 ppb/d to Holstein steers for 4 months during late fattening period can improve some blood metabolites and beef quality regarding fatty acid composition by increasing PUFA and gamma-linoleate compositions of beef. However, further research is warranted to validate the present results.
ACKNOWLEDGMENTS
Authors are thankful to Austin Thelen (Department of Communication, Oregon State University, USA) and Lindsay M. Loehden (Linfield College, USA) for their help in editing the manuscript.
Notes
CONFLICT OF INTEREST
We certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.