 |
 |
| Anim Biosci > Volume 30(1); 2017 > Article |
|
Abstract
Objective
The aim of this study was to investigate the expression of heat shock protein (HSP) 90, 70, and 60 in chicken muscles and their possible relationship with quality traits of meat.
Methods
The breast muscles from one hundred broiler chickens were analyzed for drip loss and other quality parameters, and the levels of heat shock protein (HSP) 90, 70, and 60 were determined by immunoblots.
Results
Based on the data, chicken breast muscles were segregated into low (drip loss≤5%), intermediate (5%<drip loss<9.5%) and high (drip loss≥9.5) drip loss groups. The expression of HSP90 and HSP60 were significantly lower in the high drip loss group compared to that in the low and intermediate drip loss group (p<0.05), while HSP70 was equivalent in abundance in all groups (p>0.05).
Muscle is comprised of about 75% water at rigor, the majority of which is held within the myofibrils [1]. Water-holding capacity (WHC) is defined as the ability of fresh meat to retain its own water and can be measured as drip loss. Incidence of high drip loss not only represents an economic problem for industries due to the significant weight loss of the carcass, but also influences quality attributes like tenderness and appearance of the meat. The drip loss in fresh meat is believed to be influenced by the genetics, rate of post mortem pH decline and stress [2], and structurally originates from the shrinkage of myofibrils, the permeability change of the cell membrane and the cytoskeletal protein degradation [1]. However, the mechanisms regarding drip formation in meat postmortem is not fully understood.
In response to cell damage, a set of proteins can be synthesized to protect cells. Heat shock proteins (HSPs) are molecular chaperones either expressed constitutively or inductively in regulating cellular homeostasis and promoting cell survival [3]. Mammalian HSPs have been classified into five families according to their molecular size: HSP100, HSP90, HSP70, HSP60, and small HSPs. Several studies have reported the differential expression of HSPs, specifically small HSPs, in muscle with variable pH, tenderness, color and flavor [4,5].
The relationship of HSPs and water retention of meat is still not clear and little work has been published. A high level of HSP70 has been suggested to be a potential marker for moderate or good WHC of pork [6], and the transportation of pigs resulted in decreased HSPs levels and higher water loss [7]. It was hypothesized in these findings that high levels of HSPs might be advantageous for protection against cell death and cell membrane damage. This would lead us to infer that HSPs are correlated with the WHC of the meat. Our earlier studies have preliminarily found HSP90 could bind with membrane phospholipid and associated with some quality traits of pig muscles [8,9], therefore the purpose of this study is to evaluate variations in the expression of HSPs (HSP90, 70 and 60) in chickens and establish their relationship with meat quality to understand better their possible roles in the improvement of WHC of meat products and the biological mechanisms involved.
One hundred yellow feather broiler chickens (Gallus gallus domesticus) six-week of age with the average weight of around 1.25 kg were obtained from a commercial processing plant where they were slaughtered by bleeding from a unilateral neck cut severing the left carotid artery and jugular vein (Jiangsu Lihua Animal Husbandry Co., Ltd, Nanjing, China). Two skinless, de-boned breast fillets (Pectoralis major) were taken immediately from each carcass and placed into a plastic bag on ice. Around 6 g muscles were cut and kept at 4°C for the measurement of pH at 45 min postmortem, 5 g muscle was collected from one end of each sample and stored at −20°C for the quantitation of HSP, and the residual muscle was subjected to measurements of color, cooking loss, drip loss and shear force on the sampling day after the measurement of initial pH. All measurements were performed in triplicate.
Two grams of muscle, taken at 45 min, was homogenized at 5,000 rpm with an Ultra Turrax homogenizer (T25, IKA, Labortechnik, Staufen, Germany) in 18 mL distilled water, and the pH of the homogenate was measured using a pH meter equipped with an electrode (FE-20, Mettler Toledo, Shanghai, China). The pH meter was standardized by a two point method against buffer standards of pH 6.86 and pH 4.0.
The meat color (L*, a*, and b*) was measured using a Colorimeter (CR 400, Minolta, Osaka, Japan). The colorimeter was calibrated using a standard white ceramic tile before measuring each sample.
Each sample was weighted accurately prior to cooking. The sample in a polyethylene cooking bag was immersed in an 80°C water bath until reaching an internal endpoint of 75°C. After cooking, the sample was cooled to the internal temperature of room temperature and wiped with blotting paper to remove excess water, followed by weighting immediately. Cooking loss was calculated as, Cooking loss (%) = [(raw weight − cooked weight)/raw weight]×100. After measuring of cooking loss, the same muscle was then used for the determination of shear force. Shear force was determined through the application of the Meullenet-Owens razor shear test, using a texture analyzer (TVT-300XP, TexVol Instruments, Viken, Sweden) equipped with a razor blade with a height of 24 mm and a width of 8.9 mm. Muscle strips were cut across the fiber axis. The crosshead speed was set at 2 mm/s, and the test was triggered by a 10 g contact force. The shear was perpendicular to the axis of muscle fibers.
A 4 cm diameter sample was collected and weighed, followed by suspending from a steel wire hook within a polyethylene plastic bag at 4°C for 24 h. After 24 h, sample was reweighed, and drip loss was calculated as (initial weight of sample − final weight of sample)/initial weight of sample×100.
Protein from one end of Pectoralis major muscles was extracted according to Laville et al [10] with slight modifications. Approximately 2 g of frozen muscles from each sample were crushed and homogenized on ice in 20 mL of Tris-HCl buffer (100 mM Tris-HCl, pH 8.0) and a protease inhibitors cocktail (Sigma–Aldrich Corp., St. Louis, MO, USA) followed by centrifugation at 12,000 g for 10 min at 4°C. The final protein concentration was 20 mg/mL as determined with the Bradford Protein Assay Kit (A045-2, Jiancheng Bioengineering Institute, Nanjing, China). An aliquot of the supernatant was mixed with an equivalent volume of reduced sample buffer (62.5 mM Tris-HCl [pH 6.8], 10% glycerol, 2% sodium dodecyl sulphate [SDS], 5% 2-mercaptoethanol, 0.02% bromophenol blue), then heated for 5 min at 95°C. The SDS-polyacrylamide gel electrophoresis was carried out by the method of Laemmli [11] with slight modifications. Proteins were separated on 8% SDS-polyacylamide gels. Samples (15 μL) were loaded onto wells of gels and separated in a BioRad Mini PROTEAM Tetra Cell (Bio-Rad laboratories, Hercules, CA, USA). Gels were transferred to 0.45 μm polyvinylidene fluoride (PVDF) membrane (Millipore Corp., Bedford, MA, USA) in transfer buffer (25 mM Tris–HCl, pH 8.3, 1.4% glycine, 20% methanol) at a constant current of 2.5×membrane area mA for 1 h using a Semi-Dry Electrophoretic Transfer Cell (Bio-Rad Laboratories, USA). The PVDF membrane was blocked with 5% non-fat milk for 2 h diluted with TBS-Tween (10 mM Tris–HCl, pH 8.0, 150 mM NaCl, and 0.05% Tween 20). Membranes were then washed three times with tris-buffered saline Tween (TBST) and then incubated with the primary antibody overnight at 4°C. Primary antibodies were used at the following concentrations in TBST: mouse HSP90 (Stressgen, Victoria, BC, Canada; SPA-830), 1:1,000; mouse HSP70 (Stressgen, Canada; SPA-820), 1:1,000; mouse HSP60 (Abcam, Cambridge, UK; LK-1), 1:2,000; and mouse glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Earthox, San Francisco, CA, USA; E021010-01), 1:4,000. After washing, the membranes were incubated with Horseradish peroxidase-labeled anti-mouse secondary antibodies for 2 h at room temperature at 1:5,000 dilutions in TBST. After three 5-min washes, the PVDF membranes were visualized with Diaminobenzidine for 30 min. Images of the PVDF membranes were captured by Gel Imager and then the intensities of bands in each lane were quantified using Quantity One software (Bio-Rad Laboratories, USA). The relative value of protein band intensity was calculated as intensity of the HSP band in each lane in comparison to the intensity of the GAPDH band.
Statistical analysis of the differences between each group was evaluated by one-way analysis of variance using the SPSS 18.0. The correlation coefficient was estimated with Pearson correlation coefficient option of SPSS 18.0. Differences were regarded as significant at p<0.05. All data were expressed as mean±standard error.
Drip loss of chicken breast muscle was measured in a wide range of 3.87 to 11.1. Based on the data obtained, three drip loss groups were identified: low (drip loss≤5%), intermediate (5%<drip loss<9.5%) and high (drip loss≥9.5), which was accounted for around 30%, 56.7%, 13.3% respectively in a hundred chickens. Six chickens were randomly selected in each group and their quality parameters are presented in Table 1. Mean drip loss in the high, intermediate and low drip loss groups was 10.04%, 7.21%, and 4.75% respectively. The average drip loss of the high drip loss group was more than 2 fold that of low drip loss group.
Drip loss is believed to be influenced by rate and extent of postmortem glycogen metabolism and correlated with rate of pH decline and cooking loss in some studies [12]. However, the correlation of pH and drip loss varied between studies, Otto et al [13] used different methods for determination drip loss and found the drip loss was not significantly correlated with pH and other quality traits. Other studies also showed there is often a poor correlation between the water holding capacity of raw meat, the water lost during cooking and other quality parameters [6,14]. In the present study there were no significant difference in pH, color and shear force among these groups (p>0.05). The cooking loss of high drip group was higher than that of the low drip group, but the difference was not significant (p>0.05). The drip loss is a complex attribute of meat which is influenced by animal breeds, muscle types and chilling temperature etc.; however, higher drip loss was reported to occur during early postmortem which corresponds with decreased water holding capacity [15], and the variations in drip loss could be primarily attributed to pre-slaughter stress [16].
As shown in Figure 1, chicken breast muscles with high drip loss showed lower levels of HSP90 compared to that of low and intermediate drip loss groups. The amount of HSP90 in low drip loss group was not significantly different with that in intermediate drip loss group (p>0.05). The present results were in accordance with our previous studies in pig muscles that higher levels of HSP90 were associated with better WHC [9].
The HSP70 family of HSPs contains multiple homologs including HSP72, HSP73, and Grp78. The HSP73 which could be both constitutively expressed and stress-induced was studied in this research. HSP70 was more abundantly expressed as compared to HSP90 and HSP60, but the levels of HSP70 were equivalent for all three drip loss groups (Figure 2). In contrast, it was found there was a correlation between increased drip loss after transport stress and a decline in HSP70 abundance [7], and this is also supported by Luca et al [6], who proposed that HSP70 was more abundant in high WHC pig muscles. However, van Laack et al [17] reported that expression of both cognate and inducible forms of HSP70 were not correlated with stress and drip loss of pig muscles.
Similar with the expression pattern of HSP90, lower levels of HSP60 were observed in high drip loss group, and the difference in HSP60 between low and intermediate group chicken muscles was not apparent (Figure 3). It is also shown in statistical analysis that the level of HSP60 was positively correlated with HSP90 (p<0.05) and negatively correlated with drip loss (p<0.01), while HSP70 amount was not correlated with HSP90, HSP60, and drip loss (p>0.05) (Table 2).
HSPs are known as stress proteins triggered by various environmental stress conditions, such as infection, hypoxia, starvation and water deprivation [3]. They play important roles in maintaining cellular homeostasis and integrity. It is accepted in general that the animals exposed to more intense pre-slaughter stress had poorer meat quality [18], but some studies found animals subjected to longer lairage or transport time resulted in lower drip loss and improved meat quality compared to short time of stress [19]. The possible mechanism may be the enhanced adaptability to stress and increased synthesis of HSPs in animals to limit damage. It was suggested that repeated mild stress induced up-regulation of HSP expression and synthesis could have beneficial effects on cellular function and survival [20]. Different HSPs may differ in their capabilities to protect tissue in response to stress [21]. The response to heat stress also displayed tissue-specific patterns, whereas heart was the most sensitive tissue and muscle was the least responsive to heat challenges [22]. The synthesis of HSP90 and HSP60 may vary between animals due to slaughter and pre-slaughter stress, and a decrease in HSP90 and HSP60 expression might be disadvantageous to the recovery of cellular function and therefore lead to lower water retention of meat.
HSPs are present not only in the cytosol and in the cellular organelles, but also on the surface or within the cellular membranes [23]. Disintegration of cellular membranes and increased permeability have been recognized postmortem and could have considerable influence on WHC [1], while the association of HSPs with membranes can re-establish the fluidity and permeability and restore membrane functionality [23]. HSP90, HSP70, and HSP60 have been reported to be associated with cell membranes [8,24,25]. HSP70 was shown to bind with acidic membrane phospholipids and HSP90 had the ability to bind with neutral phospholipids [8,25]. During postmortem the phospholipase A2 (PLA2) could catalyze the hydrolysis of membrane phospholipids producing lysophospholipids which leads to the loss of osmolytes and cell water, but in our previous studies HSP90 could protect phospholipid from PLA2 hydrolysis and inhibit Lysophospholipid formation [8,26]. Since the postmortem muscle acidification is accompanied with the inversion of acidic phospholipids to external and neutral phospholipids to internal [27], HSPs may play different roles in the membrane function and drip formation.
Several authors have reported that degradation of cytoskeletal proteins such as desmin was associated with the water holding capacity of meat [28,29]. A high level of desmin degradation resulted in less shrinkage of the muscle cells and ultimately contributed to improving WHC [28,29]. Degradation of these proteins including desmin is mainly due to activation of μ-calpain [29], and a recent finding showed that HSP90 interacted with μ-calpain and maintained its activity [30]. Cytoskeletal proteins play a critical role in immobilizing water in muscle, and HSPs could be involved in different pathways and form complex with a number of proteins to modulate their functions [27]. The present results indicated high levels of HSP90 and HSP60 were associated with intermediate and low drip loss of chicken muscles while HSP70 expression was not found to be correlated with drip loss of muscles. Water loss from the muscle is impacted by a variety of the structural changes of muscle, thus it is still to be explored the contribution of different HSPs on water retention of meat and the actual mechanisms.
ACKNOWLEDGMENTS
This study was supported by National Natural Science Foundation of China (31271891), National Natural Science Foundation of China (31401560), Innovation of Agricultural Science and Technology of Jiangsu Province (CX(14)5058).
Figure 1
Western blot of heat shock protein (HSP) 90 (A), glyceraldehyde-3-phosphate dehydrogenase (B) in high (n = 6), intermediate (n = 6) and low (n = 6) drip loss grouped chicken muscles, and relative intensities of HSP90 (C). Error bars are standard error of the mean. Different letters indicate significant difference at p<0.05.
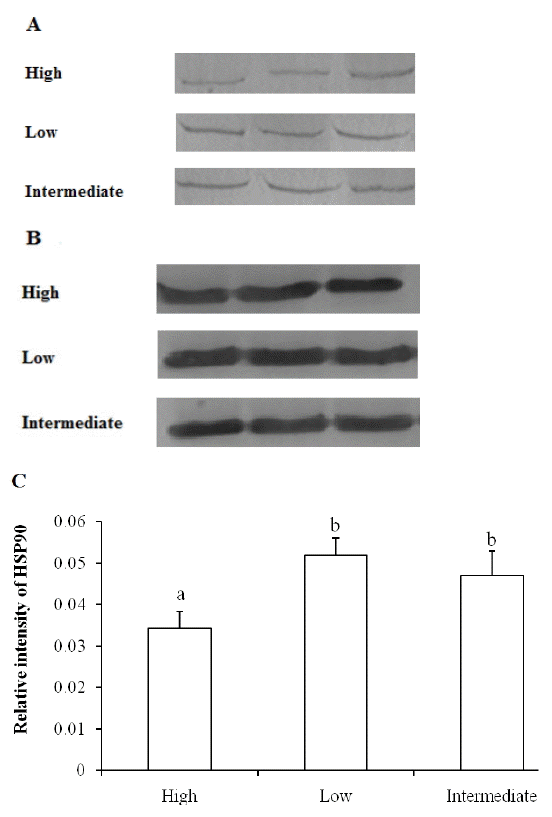
Figure 2
Western blot of heat shock protein (HSP) 70 (A), glyceraldehyde-3-phosphate dehydrogenase (B) in high (n = 6), intermediate (n = 6) and low (n = 6) drip loss grouped chicken muscles, and relative intensities of HSP70 (C). Error bars are standard error of the mean.

Figure 3
Western blot of heat shock protein (HSP) 60 (A), glyceraldehyde-3-phosphate dehydrogenase (B) in high (n = 6), intermediate (n = 6) and low (n = 6) drip loss grouped chicken muscles, and relative intensities of HSP60 (C). Error bars are standard error of the mean. Different letters indicate significant difference at p<0.05.
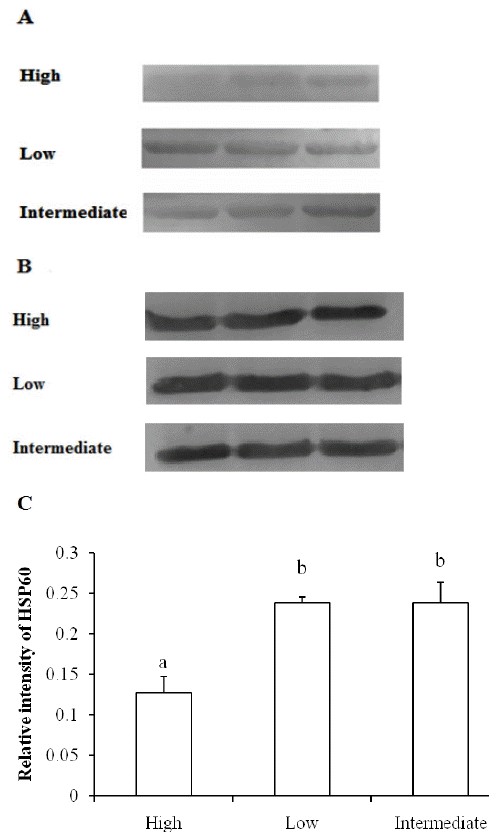
Table 1
Meat quality of chicken muscles in high, low and intermediate drip loss groups
REFERENCES
1. Huff-Lonergan E, Lonergan SM. Mechanisms of water-holding capacity of meat: The role of postmortem biochemical and structural changes. Meat Sci 2005; 71:194–204.


2. Fischer K. Drip loss in pork: influencing factors and relation to further meat quality traits. J Anim Breed Genet 2007; 124:12–8.

3. Sreedhar AS, Csermely P. Heat shock proteins in the regulation of apoptosis: new strategies in tumor therapy. Pharmacol Ther 2004; 101:227–57.


4. Pulford DJ, Dobbie P, Fraga Vazquez S, et al. Variation in bull beef quality due to ultimate muscle pH is correlated to endopeptidase and small heat shock protein levels. Meat Sci 2009; 83:1–9.


5. Lomiwes D, Farouk MM, Frost DA, Dobbie PM, Young OA. Small heat shock proteins and toughness in intermediated pHu beef. Meat Sci 2013; 95:472–9.


6. Luca AD, Mullen AM, Elia G, Davey G, Hamill RM. Centrifugal drip is an accessible source for protein indicators of pork ageing and water-holding capacity. Meat Sci 2011; 88:261–70.


7. Yu J, Tang S, Bao E, et al. The effect of transportation on the expression of heat shock proteins and meat quality of M. longissimus dorsi in pigs. Meat Sci 2009; 83:474–8.


8. Wang DY, Zhang MH, Liu F, Zhu YZ, Xu WM. Purification and characterization of a phosphatidylcholine-binding protein from duck Biceps femoris muscle. Anim Prod Sci 2014; 54:194–9.

9. Zhang M, Wang D, Geng Z, et al. The level of heat shock protein 90 in pig Longissimus dorsi muscle and its relationship with meat pH and quality. Food Chem 2014; 165:337–41.


10. Laville E, Sayd T, Morzel M, et al. Proteome changes during meat aging in tough and tender beef suggest the importance of apoptosis and protein solubility for beef aging and tenderization. J Agric Food Chem 2009; 57:10755–64.


11. Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970; 227:680–5.


12. Warriss PD. The relationship between pH45 and drip in pig muscle. Int. J Food Sci Technol 1982; 17:573–8.

13. Otto G, Roehe R, Looft H, Thoelking L, Kalm E. Comparison of different methods for determination of drip loss and their relationships to meat quality and carcass characteristics in pigs. Meat Sci 2004; 68:401–9.


14. Hughes JM, Oiseth SK, Purslow PP, Warner RD. A structural approach to understanding the interactions between colour, water-holding capacity and tenderness. Meat Sci 2014; 98:520–32.


15. Kristensen L, Purslow PP. The effect of ageing on the water-holding capacity of pork: Role of cytoskeletal proteins. Meat Sci 2001; 58:17–23.


16. Young JF, Bertram HC, Oksbjerg N. Rest before slaughter ameliorates pre-slaughter stress-induced increased drip loss but not stress-induced increase in the toughness of pork. Meat Sci 2009; 83:634–41.


17. Van Laack RL, Faustman C, Sebranek JG. Pork quality and the expression of stress protein Hsp70 in swine. J Anim Sci 1993; 71:2958–64.


18. Warriss PD, Brown SN, Gade PB, et al. An analysis of data relating to pig carcass quality and indices of stress collected in the European Union. Meat Sci 1998; 49:137–44.


19. Pérez MP, Palacio J, Santolaria MP, et al. Effect of transport time on welfare and meat quality in pigs. Meat Sci 2002; 61:425–33.


20. Minois N. Longevity and aging: beneficial effects of exposure to mild stress. Biogerontology 2000; 1:15–29.


21. Yu J, Bao E, Yan J, Lei L. Expression and localization of Hsps in the heart and blood vessel of heat-stressed broilers. Cell Stress Chaperon 2008; 13:327–35.



22. Xie J, Tang L, Lu L, et al. Differential expression of heat shock transcription factors and heat shock proteins after acute and chronic heat stress in laying chickens (Gallus gallus). PloS ONE 2014; 9:e102204



23. Horvath I, Multhoff G, Sonnleitner A, Vigh L. Membrane-associated stress proteins: More than simply chaperones. Biochim Biophys Acta 2008; 1778:1653–64.


24. Pfister G, Stroh CM, Perschinka H, et al. Detection of HSP60 on the membrane surface of stressed human endothelial cells by atomic force and confocal microscopy. J Cell Sci 2005; 118:1587–94.


25. Harada Y, Sato C, Kitajima K. Complex formation of 70-kDa heat shock protein with acidic glycolipids and phospholipids. Biochem Biophys Res Commun 2007; 353:655–60.


26. Lambert IH, Nielsen JH, Andersen HJ, Ortenblad N. Cellular model for induction of drip loss in meat. J Agric Food Chem 2001; 49:4876–83.


27. Ouali A, Herrera-Mendez CH, Coulis G, et al. Meat tenderisation and muscle cell death, two highly related events. Tehnoligija Mesa 2007; 48:1–15.
28. Rowe LJ, Huff-Lonergan E, Lonergan SM. Desmin degradation influences water-holding capacity and tenderness of fresh pork. J Anim Sci 2001; 79:443
- TOOLS
-
METRICS

- Related articles
-
Expression of heat shock protein genes in Simmental cattle exposed to heat stress2023 May;36(5)





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print




