Effects of low NaNO2 and NaCl concentrations on Listeria monocytogenes growth in emulsion-type sausage
Article information
Abstract
Objective
The objective of this study was to evaluate the effect of combinations of NaNO2 and NaCl concentrations on Listeria monocytogenes (L. monocytogenes) growth in emulsion-type sausage.
Methods
Emulsion-type sausages formulated with different combinations of NaNO2 (0 and 10 ppm) and NaCl (1.00%, 1.25%, and 1.50%) were inoculated with a five-strain L. monocytogenes mixture, and stored at 4°C, 10°C, and 15°C, under aerobic or vacuum conditions. L. monocytogenes cell counts were measured at appropriate intervals, and kinetic parameters such as growth rate and lag phase duration (LPD) were calculated using the modified Gompertz model.
Results
Growth rates increased (0.004 to 0.079 Log colony-forming unit [CFU]/g/h) as storage temperature increased, but LPD decreased (445.11 to 8.35 h) as storage temperature and NaCl concentration increased. The effect of combinations of NaCl and low-NaNO2 on L. monocytogenes growth was not observed at 4°C and 10°C, but it was observed at 15°C, regardless of atmospheric conditions.
Conclusion
These results indicate that low concentrations of NaNO2 and NaCl in emulsion-type sausage may not be sufficient to prevent L. monocytogenes growth, regardless of whether they are vacuum-packaged and stored at low temperatures. Therefore, additional techniques are necessary for L. monocytogenes control in the product.
INTRODUCTION
Listeria monocytogenes (L. monocytogenes) is a foodborne bacterium found in processed meat products; it can contaminate the products during post-processing steps such as slicing, packaging and handling [1,2]. The pathogen causes foodborne illness following intake of meat products, including those that are formulated with NaNO2 and NaCl [3]. NaCl is added to processed meat products to improve flavor and water-holding capacity and preservation [4]. NaNO2 is used as a color-fixing agent in processed meat products, and also has an anti-microbacterial effect, particularly for inhibiting germination of Clostridium botulinum [5,6]. Combinations of NaCl (2.7% w/v) and NaNO2 (180 mg/L) had an inhibitory activity of bacteria growth [7]. Xi et al [8] showed that growth of L. monocytogenes was inhibited by high NaNO2 (200 ppm), and other studies have also reported that NaNO2 affects L. monocytogenes growth by decreasing the growth rate and increasing lag time [9,10].
In general, food additives improve the safety and taste of foods. However, some people suggested that unfavorable chemicals could be produced during curing [11]. A study by Ministry of Food and Drug Safety (MFDS) [12] presented that 54.6% of consumers believe that food additives have negative effects on human health. In addition, Bedale et al [13] presented that consumers had fear against chemical food additives and antimicrobials. Specifically, consumers believe that NaNO2 forms N-nitroso compounds, which are human carcinogens, in an acidic environment [14,15]. Thus, in response to the fact that consumers prefer not to have the food additive in their foods, many companies have recently started to produce meat products with NaNO2 replacements and low NaNO2 concentration, and celery powder, Swiss chard powder and rosemary have been used as NaNO2 replacements [16,17]. However, these replacements lowered quality for flavor and meat color [8,14,18].
Therefore, the objective of this study was to determine the kinetic behavior of L. monocytogenes in emulsion-type sausage, which was formulated with either low concentrations of NaNO2 and NaCl or no NaNO2 and NaCl, instead of using NaNO2 replacement.
MATERIALS AND METHODS
Inoculum preparation
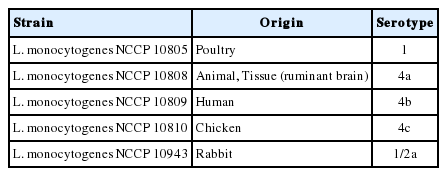
L. monocytogenes strains NCCP10805, NCCP10808, NCCP10809, NCCP10810, and NCCP10943, which were isolated from meats and human (Table 1), were cultured in 10 mL nutrient broth plus 0.6% yeast extract (NBYE; Beckton, Dickinson, and Company, Sparks, MD, USA) at 30°C for 24 h. Then, 0.1 mL portions of these cultures were subcultured in 10 mL fresh NBYE at 30°C for 24 h. The subcultures were harvested by centrifugation at 1,912×g for 15 min at 4°C, washed twice with phosphate-buffered saline (PBS; pH 7.4; 0.2 g of KH2PO4, 1.5 g of Na2HPO4·7H2O, 8.0 g of NaCl, and 0.2 g of KCl in 1 L distilled water), and resuspended in 10 mL PBS. Each strain was mixed and serially diluted in PBS to obtain 5 to 6 Log colony-forming unit (CFU)/mL of L. monocytogenes for use as an inoculum.
Emulsion-type sausage preparation
Fresh pork and pork back fat were purchased from a local butcher shop 24 h after slaughter, and the connective tissue and extra fat layer were removed. Meat and fat were ground using a meat chopper (PM-70, Mainca, Barcelona, Spain) equipped with an 8-mm plate, and emulsified for 6 min using a silent cutter (MSK 760 H II, Mado, Dornhan, Germany). Emulsion-type sausages were then prepared as described in Table 2. Each treatment mixture was stored at 4°C for 1 h to enhance the binding strength and stability of the mixture [19]. Thirty grams of emulsion was filled into a collagen casing (25 mm diameter) using a filling machine (Konti A50, Frey, Herbrechtingen, Germany). For pasteurization, the emulsion-type sausage samples were heated at 75°C for 40 min and then chilled. The emulsion-type sausages were then vacuum-packed and heated again at 80°C for 15 min; subsequently, they were stored at 4°C until use.
Inoculation and microbial analysis
The emulsion-type sausage was cut into 25 g portions. Thirty sausage samples were dipped into a sterile plastic container containing inoculum at 2 to 3 Log CFU/mL which was set up to observe sufficient growth, and stirred gently for 2 min. After dipping, the samples were air-dried under a biosafety cabinet for 15 min to allow the L. monocytogenes cells to attach; then, they were transferred to vacuum bags. The bags were either simply sealed (aerobic storage) or vacuum-packaged (vacuum storage). The sealed samples were stored at 4°C for 1,440 h, at 10°C for 528 h and at 15°C for 192 h. During storage, L. monocytogenes cell counts were measured on PALCAM medium base agar (Beckton, Dickinson, and Company, USA) at appropriate time intervals (11 to 23 times). Sodium nitrite residues were determined according to Korean Food Standards [20].
Calculation of kinetic parameters
To calculate kinetic parameters such as growth rate (Log CFU/g/h) and lag phase duration (LPD; h), L. monocytogenes growth data for each storage condition were fitted to the modified Gompertz model [21,22] as follows:
Where A is the lower asymptotic line of the growth curve as t decreases to zero, B is the growth rate at time M, C is the difference between the upper asymptotic line of the growth curve (Nmax) minus the lower asymptotic line (N0), and M is the time at which the growth rate is maximum. According to this equation, growth rate and LPD can be calculated by the equations shown below:
Statistical analysis
Growth rate (n = 4) and LPD (n = 4) values were analyzed using 3 (4°C, 10°C, and 15°C)×3 (1.00%, 1.25%, and 1.50% NaCl) factorial design for each NaNO2 concentrations (0 ppm and 10 ppm). The data were analyzed by the general linear model procedure using SAS version 9.3 (SAS Institute, Cary, NC, USA) for fixed effects and interactions between fixed effects. Least square (LS) means between the interactions were compared by a pairwise t-test at the significance level of alpha = 0.05.
RESULTS AND DISCUSSION
The growth rates of L. monocytogenes in emulsion-type sausage increased under aerobic and vacuum storage as the temperature increased, regardless of NaNO2 and NaCl concentrations (Figures 1 and 2). To determine the kinetic behavior of L. monocytogenes in the emulsion-type sausage, kinetic parameters for the pathogen were calculated using the modified Gompertz model. The modified Gompertz model was fitted to the L. monocytogenes cell counts, and R2 values were 0.944 to 0.987 for aerobic storage, and 0.933 to 0.992 for vacuum storage. These results indicate that the model is appropriate for calculating the kinetic parameters of L. monocytogenes in emulsion-type sausage.

Bacterial populations of Listeria monocytogenes cell counts in emulsion-type sausage in aerobic storage.

Bacterial populations of Listeria monocytogenes cell counts in emulsion-type sausage in vacuum storage.
For aerobic storage, higher LPD values (245.56 to 445.11 h; p<0.05) were obtained at 4°C than 10°C (22.76 to 47.24 h) or 15°C (8.35 to 23.33 h) storage. Growth rate values increased as storage temperature increased. At 4°C, the growth rate (0.004 to 0.008 Log CFU/g/h) of L. monocytogenes was very low (p<0.05), as compared to those (0.028 to 0.079 Log CFU/g/h) at 10°C and 15°C, regardless of NaNO2 and NaCl concentration (Table 3). Even though the growth rate of L. monocytogenes was very low, the pathogen gradually grew at 4°C (Table 3). At 4°C and 10°C, a combination effect of NaNO2 and NaCl on L. monocytogenes was not observed, but at 15°C, growth rates (0.043 to 0.050 Log CFU/g/h) were lower (p<0.05) in 10-ppm NaNO2 treated samples than in 0-ppm NaNO2 treated samples (0.065 to 0.079 Log CFU/g/h), regardless of NaCl concentration, indicating that under aerobic storage a combiation effect of NaNO2 and NaCl on L. monocytogenes is temperature-dependent (Table 3). Because the growth at 4°C and 10°C was too slow to show antimicrobial effect, combinations of NaNO2 and NaCl on L. monocytogenes may not be observed at low temperature. The higher temperatures had higher Nmax values (4°C: 7.0 to 7.6 Log CFU/g, 10°C: 8.0 to 8.6 Log CFU/g, 15°C: 8.6 to 8.9 Log CFU/g) (Table 3). These results suggest that a low NaNO2 concentration may allow L. monocytogenes growth even in NaCl combination, and therefore, alternative or additional techniques are necessary to inhibit L. monocytogenes growth in the emulsion-type sausage formulated with low NaNO2 and NaCl concentrations.

The growth parameters estimated by the modified Gompertz model for Listeria monocytogenes on emulsion-type sausage as a function of NaNO2 and NaCl concentration at 4°C, 10°C, and 15°C in aerobic storage
For vacuum storage, LPD values were higher (p<0.05) at 4°C (114.23 to 442.33 h) than at 10°C (24.48 to 90.25 h) or 15°C (9.26 to 17.40 h). As shown for aerobic storage, growth rates (0.003 to 0.005 Log CFU/g/h) at 4°C were very low (p<0.05), compared to those (0.019 to 0.072 Log CFU/g/h) at 10°C and 15°C, regardless of NaNO2 and NaCl concentration (Table 4). In addition, no differences in growth rates were observed for the different NaNO2 and NaCl concentrations at either 4°C or 10°C (Table 4). However, at 15°C, growth rates (0.041 to 0.048 Log CFU/g/h) were lower (p<0.05) in 10 ppm NaNO2 than in 0 ppm NaNO2 (0.058 to 0.072 Log CFU/g/h), and NaNO2 growth rates decreased as NaCl concentration increased in 0 ppm (Table 4), which was an opposite result for aerobic storage at 15°C. Nmax values were lower (p<0.05) at 4°C (7.3 to 7.7 Log CFU/g) than at 10°C (8.0 to 9.0 Log CFU/g) or 15°C (8.5 to 8.7 Log CFU/g) (Table 4). These results indicate that low NaNO2 concentration is not sufficient to inhibit L. monocyogenes growth completely.
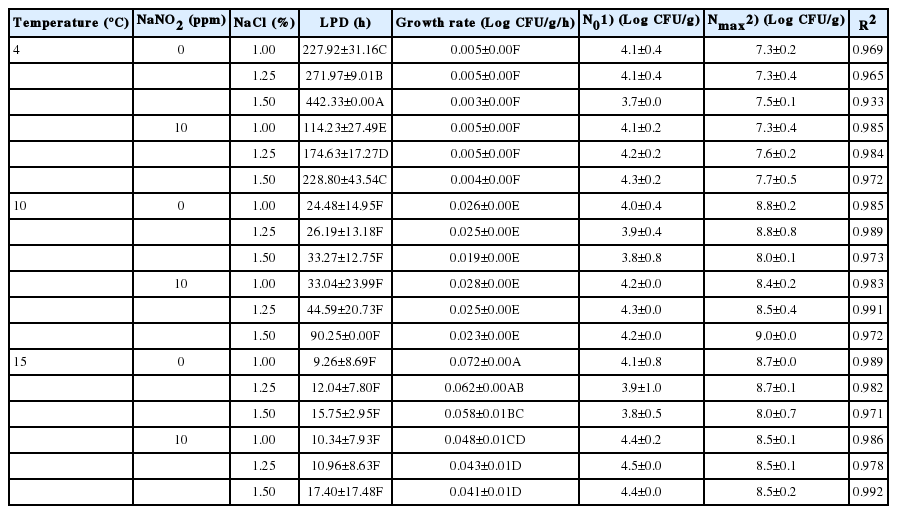
The kinetic parameters estimated by the modified Gompertz model for Listeria monocytogenes on emulsion-type sausage as a function of NaNO2 and NaCl concentration at 4°C, 10°C, and 15°C in vacuum storage
Growth rates were similar between aerobic and vacuum storage (Tables 3 and 4). Taken together, these results suggest that 10 ppm NaNO2 may not completely inhibit L. monocytogenes growth even in combination with NaCl and in vacuum storage and even when samples are stored at 4°C.
In other literature, antimicrobial effects of NaNO2 and NaCl were observed. Doyle and Glass [23] showed that generation time of L. monocytogens became longer in broth media supplemented high NaNO2 and NaCl. Jo et al [24] showed that there is a combination effect of low NaNO2 with NaCl on Pseudomonas spp. in processed meat products. Also, Lee et al [25] showed that the growth of Enterococcus spp. was also inhibited by added NaNO2 and NaCl. Gill and Holley [7] found that pathogenic bacteria such as Staphylococcus aureus and L. monocytogenes were inhibited by NaNO2 not by NaCl. In addition, single-effect of NaNO2 and NaCl was identified in several Gram-negative bacteria related to meat (Salmonella Typhimurium, Escherichia coli, Serratia grimesii, and Shewanella putrefaciens), but multi-effect was observed in only S. Typhimurium and S. putrefaciens. Lactobacillus which cause spoilage in processed meat produtcs [26,27] was inhibitied by a combination of NaNO2 and NaCl in frankfurters [28]. Also, Sallam and Samejima [29] showed that NaCl-treated ground beef supplemented with sodium lactate could delay the growth of lactic acid bacteria and Enterobacteriaceae at refrigerated storage condition. The sporulation of Clostridium perfringens and production of C. perfringens enterotoxin were also inhibited by nitrate salts as suppression of gene expression [30]. These results indicate that antimicrobial effect of NaNO2 and NaCl was clearly observed, and a combination effect of NaNO2 and NaCl is bacteria dependent.
In conclusion, our studies suggest that emulsion-type sausages that contain low NaNO2 levels, as preferred by consumers, are not safe for L. monocytogenes growth even under NaCl combination, regardless of atmospheric storage conditions. Therefore, aleternative or additional techniques are necessary for L. monocytogenes control in the product.
ACKNOWLEDGMENTS
This work was carried out with the support of “Cooperative Research Program for Agriculture Science & Technology Development (Project No. PJ009237)” Rural Development Administration, Republic of Korea.
Notes
CONFLICT OF INTEREST
We certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.