Blood biochemical parameters and organ development of brown layers fed reduced dietary protein levels in two rearing systems
Article information
Abstract
Objective
An experiment was conducted to evaluate the effect of different levels of crude protein (CP) and two rearing systems (cage and floor), on blood parameters and digestive and reproductive organ development of brown laying hens.
Methods
A total of 400 Hisex Brown laying hens between 30 and 45 weeks of age were distributed in a completely randomized design and a 2×4 factorial arrangement, with main effects including two rearing systems (cage and floor) and levels of CP (140, 150, 160, and 180 g/kg), in a total of eight treatments and five replicates of 10 birds each with initial body weight of 1,877 g (laying hen in cage) and 1,866 g (laying hens in floor). The parameters evaluated were plasma total protein, albumin, uric acid, total cholesterol, relative weights of oviduct, abdominal fat, liver, gizzard, crest and dewlap, length of small intestine and oviduct.
Results
The blood parameters were similar in birds reared in cage and floor systems. The birds reared on the floor showed greater small intestine and oviduct weight (%) and lower liver and pancreas weight (%). A significant interaction was observed between factors for the relative gizzard, crest and dewlap weight, serum protein, uric acid, and total cholesterol (p<0.05). The diets with 140 g/kg CP resulted in lower serum protein and lower cholesterol in birds reared in floor system, while birds reared in cage system showed no effect of CP on both parameters. Birds reared in cage and fed with 140 and 150 g/kg CP presented lower uric acid. The group of birds reared in floor system fed 180 g/kg had greater uric acid.
Conclusion
The dietary protein level can be reduced up to 140 g/kg for Hisex Brown hens (30 to 45 weeks of age) without an important effect on metabolic profile and organ development in both rearing systems.
INTRODUCTION
The rearing systems that allow laying hens to express their natural behaviors have been used to improve the hens welfare. Many countries have banned the use of conventional cages in laying hen production. The benefits of furnished and open systems are reduction of the prevalence of keel-bone fractures [1] and reduction of fearfulness [2]. Furthermore, the improvement of egg production and better egg internal and external quality [3,4] has been related as the benefits of open systems, like a floor system. The eggs produced in conventional cages compared with those laid in free-range farming conditions, presented higher concentrations of total lipids, cholesterol, and gross energy [5]. It has been reported that eggs produced by birds in open systems present a higher yolk percentage compared to cage system [3,6], and that the egg mineral content is affect by rearing system as well [7]. In this way, egg composition is affected by the system, therefore the blood parameters of laying hens that are related to egg formation could be affect too. Reducing space allowance in or eliminating access to a nest box results in disruption of biological function [8]. Based on this, the information about the metabolic and organ development effects in laying hens raised in alternative systems must be improved.
In addition to replacement of rearing system, poultry diets have been formulated based on the use of digestible amino acids, considering the ideal protein concept. It is reported that the reduction of dietary protein from 17% to 15.7% with amino acid supplementation is enough to maintain performance of Brown laying hens [9]. The rearing system affects both the performance of hens and the egg quality characteristics [10], that are associated to crude protein (CP) requirements by birds. Therefore, the reduction of dietary protein should be studied in different rearing systems, to determine the rearing system affect the nutritional requirements of birds. According to de Almeida Brainer et al [11] hens housed in free-range systems have a greater CP requirement for maintenance than the CP requirement for production, due to its lower productive potential and greater caloric expenditure, given the physical activities that occur under semi-intensive management.
Blood biochemical parameters had been used as metabolic variables to assess the welfare of poultry [12]. The behavior, blood corticosterone and blood biochemical parameters in laying hens change during the stress [13,14]. The blood biochemical parameters provide information about immune system of birds, which is associated with environmental conditions, and can aid in the assessment of rearing systems. In addition, the digestive and reproductive organs development, and sexual characteristics (crest and dewlap) can be used to assess the birds’ metabolism and development.
The biochemical composition of the blood plasma reflects the metabolic situation of the tissues, making it possible to evaluate changes in the organ’s functions and the adaptation of the animal to nutritional and physiological challenges, in some cases, imposed by stressful situations [15]. Hens reared in floor systems are more challenged by microorganisms than those reared in cage systems. A trend toward increased bird-to-bird transmission of Salmonella enteridis was detected in the aviary and floor system compared with the cage systems [16]. In fact, Parisi et al [17] showed that free-range eggs had higher prevalence of Salmonella and Campylobacter spp. than battery cage eggs, indicating a possible challenge to hens reared in floor system. In this context, the dietary protein requirements could be different in hens under different housing conditions as the protein participates of immune system in animals.
Due to these factors and considering that less is understood about the protein requirement for laying hens reared in a floor system, we hypothesized that the replacement from conventional cages to floor can change the protein requirements of the birds. In this context, it is important to undertake research focused on the serum biochemistryl and organ development of laying hens in floor and cage systems fed different dietary CP.
This study was conducted to investigate the blood biochemical parameters, the digestive and reproductive organs development of Hisex Brown layers, from 30th to 45th weeks of age in two rearing systems, receiving reduced dietary protein.
MATERIALS AND METHODS
Animal care and study site
This experiment was performed in accordance with Ethics Committee on the Use of Animals (CEUA/UFG), under protocol 312/11. The experiment was carried out in the city of Urutaí in the state of Goiás, Brazil, Latitude: 17° 27′ 49″ S, Longitude: 48° 12′ 06″ W, Altitude: 807 m.
Animals, experimental design, and management
Four hundred Hisex Brown layers, from 30 to 45 weeks of age, were allotted in a completely randomized design and a 2×4 factorial arrangement, with main effects including two rearing systems (cage and floor) and four levels of CP (140, 150, 160, and 180 g/kg CP), with five replicates of ten birds each with initial body weight of 1,877 g (laying hen in cage) and 1,866 g (laying hens in floor).
The experimental diets were isonutritive and formulated on the ideal protein concept, according to Rostagno et al [18] (Table 1).
The floor rearing system consisted of 20 boxes with rice hulls as a litter material. Each box was equipped with a pendular drinker, a linear tube feeder and a nest. The boxes had a measurement of 2.2×1.5×3 m (length×width×height) and the floor litter had a height of 10 cm. The nest, built out of wood, had three holes (33×40×45 cm) and an elevation of 10 cm in relation to the litter line. The density of 3.3 m2/bird was adopted on the floor system. The conventional cages size was 100×37×40 cm, with four divisions of 25 cm. The density of 500 cm2/bird in the cages was adopted. During the entire experimental period, ad libitum feed and water were provided and a lighting program with 16 h light and 8 h dark period, as indicated in the Hisex Brown Management Manual. The temperature inside the poultry houses were controlled with a maximum-minimum thermometer (Incoterm, Urutaí, GO, Brazil). The average of minimum and maximum temperature of cage system were 22.3°C and 30.1°C, respectively. The average of minimum and maximum temperature of floor system were 22.5°C and 29.9°C, respectively.
Blood sampling
The blood parameters analysis was performed on the 2nd and 4th periods of egg production (37th and 45th weeks of age) and results were presented as mean of two periods.
After 6 h fasting, five hens from each treatment (one of each replication) at 37th and 45th weeks of age, totalizing ten birds per treatment, were selected to measure the blood parameters. About 5 mL of blood was collected from cardiac puncture, in tubes containing anticoagulant (sodium fluoride). The plasma was separated by centrifugation. The levels of plasmatic total protein, albumin, uric acid, and total cholesterol were determined on an automated biochemical analyzer.
Organ properties
After collecting blood, one bird per repetition was euthanized by cervical dislocation. The small intestine, liver, pancreas, gizzard, crest, dewlap, oviduct, and abdominal fat were collected and expressed in terms of relative weight per 100 g of body weight. The length of small intestine and oviduct were measured. The birds were weighed on a precision scale. The measurement of the length of the intestines was performed from the initial portion of the duodenum to the rectum, and that of the oviduct, from the infundibulum to the vagina.
Statistical analysis
The experiment was designed as a 2×4 factorial arrangement and the data were analyzed using the R program (version 2020) in the model as follows:
in which yijk = value observed in the rearing system i (i = 1, 2), level j (j = 1, 2, 3, 4), and repetition k (k = 1, 2, 3, …, 5); μ = overall mean of the experiment; ai = fixed effect of the system i (i = 1,2); bj = fixed effect of the level j (j = 1, 2, 3, 4); (ab)ij = fixed effect of the interaction between system i (i = 1, 2) and level j (j = 1, 2, 3, 4); and ɛijk = random error in the system i (i = 1, 2), level j (j = 1, 2, 3, 4), and repetition k (k = 1, 2, 3, …, 5).
Data were submitted to analysis of variance. The significance of difference among the treatments was assessed using the Scott Knott test. The significance adopted was p-value <0.05.
RESULTS
The effect of rearing system
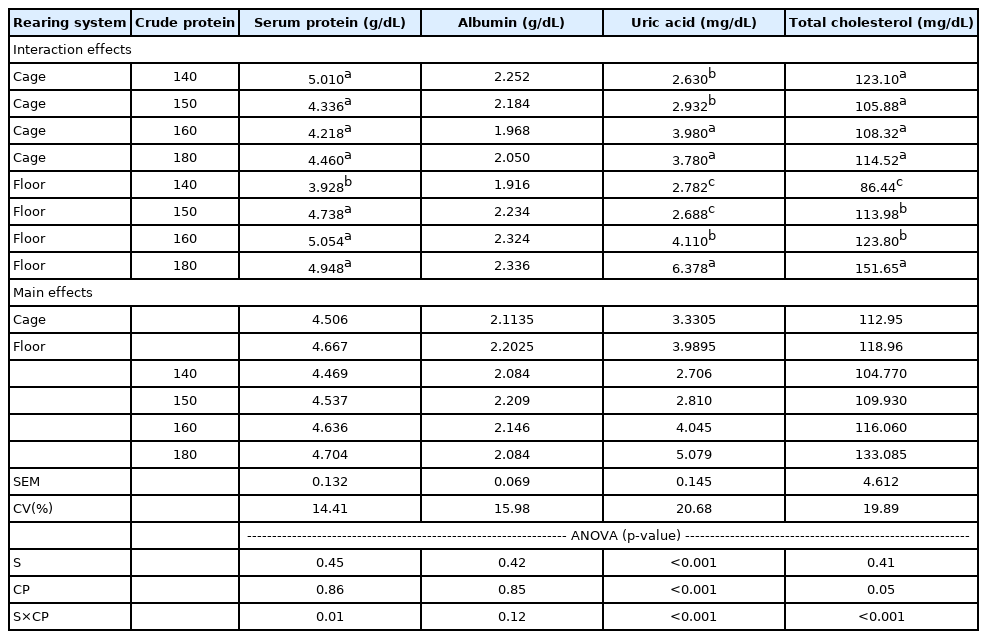
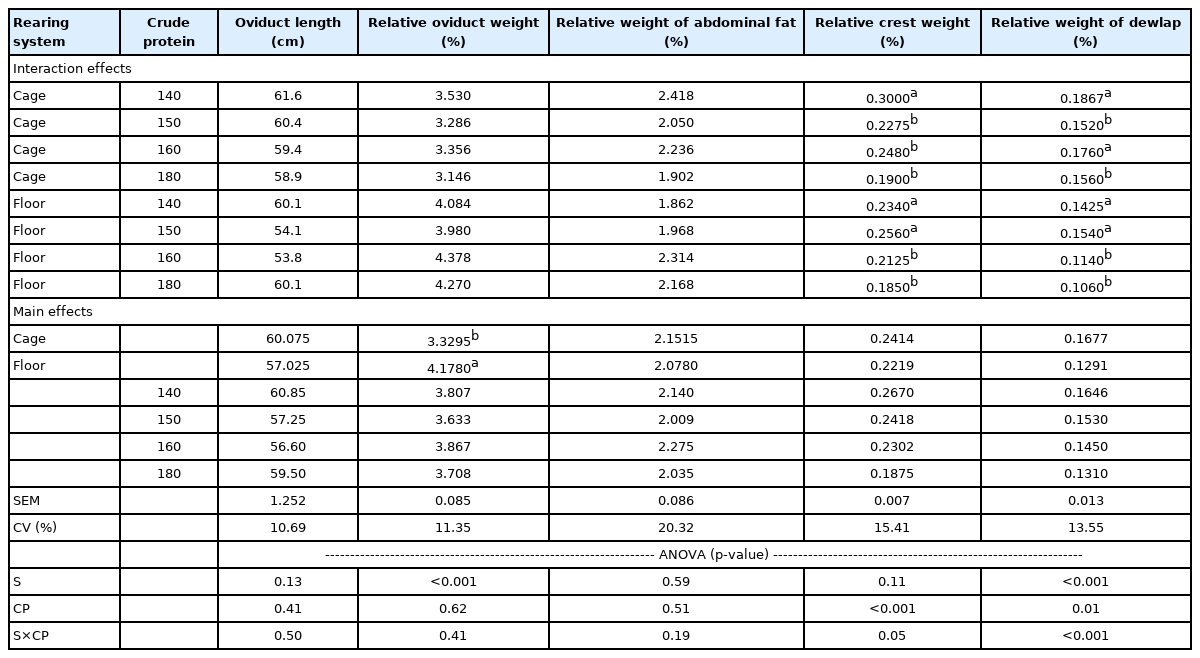
The small intestine length was not affected by the rearing system (Table 2). Compared to cage system, birds reared on the floor system showed greater small intestine (p = 0.0223, Table 2), greater oviduct (p<0.001, Table 3) and lower liver (p<0.001, Table 2) and pancreas (p = 0.0082) relative weights (Table 2). The oviduct length, the percentage of abdominal fat, the relative weight of crest and dewlap were not affected by the rearing system (Table 3). The blood parameters were similar in birds reared in cage and floor system (Table 4).

The relative weight of digestive organs (g/100 g of body weight) and small intestine length (cm) of laying hens reared different systems and fed diets with different levels of crude protein
The relative weight of reproductive organs (g/100 g of body weight) and oviduct length (cm) of laying hens reared different systems and fed diets with different levels of crude protein
The effect of crude protein content
The small intestine length was negatively affected using 140 and 180 g/kg of CP (p = 0.0127, Table 2). Relative weight of small intestine was lower in birds fed 150 and 180 g/kg of CP (p = 0.0049, Table 2). The CP did not affect the relative weight of liver, and pancreas (Table 2), oviduct length, relative weight of oviduct (Table 3) and serum albumin content (Table 4).
The statistical interaction effect for the studied factors
There were significant interactions between protein levels and raising system for the relative weight of gizzard (p = 0.0386, Table 2), crest (p = 0.0481, Table 3) and dewlap (p = 0.0067, Table 3) and to serum protein (p = 0.0138, Table 4), uric acid (p<0.001, Table 4) and total cholesterol (p = 0.0094, Table 4). Birds reared in floor system and fed diets with 160 and 180 g/kg CP presents lower relative gizzard weight, but birds reared in cage system showed no differences for this variable. The diet containing 140 g/kg CP resulted in the highest relative crest weight on birds reared in cage system. Birds reared in floor system and fed diets with 140 and 150 g/kg CP presented greater relative crest weight compared to other groups. For dewlap relative weight, birds on cage system showed greater values when fed diets with 140 and 160 g/kg CP, and hens raised in floor had showed greater relative with diets containing 160 and 180 g/kg CP.
In observation of the data presented in Table 4, the birds fed diets containing 140 g/kg CP showed lower serum protein and lower total cholesterol when reared in floor system, while the ones reared in cage system had no effect of CP on both parameters. Birds reared in cage system that were fed diets with 140 and 150 g/kg CP presented lower uric acid when compared to birds fed 160 and 180 g/kg CP rations. The group of birds reared in floor system fed 180 g/kg CP had greater uric acid, compared to the hens that received 160 g/kg CP and the lowest values were observed for the ones fed 140 and 150 g/kg CP.
DISCUSSION
The changing of rearing system from cage to floor system is a trend aimed at improving hens’ welfare. In the present study verified that laying hens reared in floor system did not show any difference in blood parameters compared to those birds reared in cage system. Blood parameters provide information on a bird’s metabolism, stress response and consequently indicate effects that can affect the performance results. It was confirmed that stress can cause elevations in blood levels of corticosterone, glucose, cholesterol, and high density lipoprotein, whereas triglycerides levels were reduced by adrenocorticotrophic hormone (ACTH) treatment [19]. Negative correlations between cholesterol content in serum and egg can indicate that eggs from hens reared in cages contained significantly more cholesterol than eggs laid by hens reared in floor pens [20]. In this context, the manipulation of serum cholesterol could result in different content in the eggs. The housing system affects the concentration of cholesterol where lower values were found on litter in comparation to cage system [21]. However, in the present experiment, the total cholesterol was similar between the rearing systems (cage and floor).
The serum content of protein, albumin, uric acid, and cholesterol are affected by age [22], pathological factors [23] and dietary additives [24]. In this experiment, a significant interaction between the dietary CP and rearing system on serum protein, uric acid, and total cholesterol of hens was observed. Exception made for albumin serum levels; the other blood parameters were affected by diets when birds were reared in floor system. On the other hand, only serum uric acid was affected by the dietary CP in birds housed in cage system. A significant interaction between the factors corresponds with the findings from Kraus et al [21] who verified that housing system, breed and age of hen affects the blood parameters with significant interaction between the factors. The birds reared in floor system presented more variation on metabolism according to dietary CP compared to birds reared in cage system. This may indicate a different demand of protein by birds in relation to housing system. Likewise, the stocking density of birds differed according to the rearing system and may contributed to differences in bird’s metabolism observed.
Birds reared in the cage system and fed diets with 160 and 180 g/kg CP presented greater levels of uric acid. This response was expected because uric acid is the method of excretion of excess of dietary nitrogen. Birds reared on the floor and fed with 180 g/kg CP produced more than 200% more uric acid than those fed with 140 g/kg CP. The excretion of uric acid increases when the diet contains an excess of CP. Besides the function of nitrogen excretion, uric acid had been related to one of the most important antioxidants in birds and is linked to their longevity [25].
The relative weight of small intestine and oviduct of laying hens reared in floor pens was greater than hens reared in cages. These results are in agreement with Yang et al [26] who reported that the weight of proventriculus, gizzard, liver, spleen, duodenum, jejunum, ileum, cecum, and rectum of free-range birds was higher than those of cagereared. Some researchers determined that organ weights were heavier in hens ranged on pasture and related that the pasture intake increased digestive organ weight [27], and fiber intake [28]. In the present study the birds were reared in floor with rice hulls as a litter, however, they did not had access to the pasture.
On the other hand, it is noticed that the floor system resulted in lower relative liver and pancreas weights of birds. These organs are responsible for enzyme production among other functions and are affected principally by diets. The liver weight has been related to stress response in laying hens [19]. Some authors verified that dry weight, moisture, protein, fat, carbohydrate, and ash contents of liver were all increased in laying hens under stress mediated by continuous infusion of ACTH [19]. The higher liver weight can indicate a metabolic disorder called fatty liver haemorrhagic syndrome (FLHS). Factors associated with husbandry practices in different production systems, such as restricted movement, increased production and temperature variations, influence hepatic lipid metabolism and predispose hens to FLHS [29].
The oviduct length, the percentage of abdominal fat, the relative crest and dewlap weights were not affected by the rearing system. These characteristics are related to reproductive performance of laying hens. Because of the stress response, the weight of the oviduct of laying hens can be reduced [19,30]. According to Wang et al [31] the elevated corticosterone levels in response to stressors may be associated with suppressed reproduction in laying hens via a possible perturbation of the hypothalamic-pituitary-gonadal axis. Therefore, based in reproductive characteristics, the birds did not present symptoms of stress according to the rearing system studied.
The reduction of dietary CP with amino acids supplementation aims to minimize the pollution with nitrogen products from poultry excretion [32]. Furthermore, the reduction of CP with amino acid supplementation improves the hens’ egg production, the small intestine villus height and promoting beneficial effects of faecal microflora [33].
In the present study, the small intestine length and relative weight of small intestine were affected by dietary CP. Both low and greater levels of CP negatively affected the intestine development. The other parameters evaluated were not affected by the dietary CP as an isolated factor, indicating that the reduction of dietary CP until 140 g/kg with amino acids supplementation can be used as a criterion for diet formulations.
Significant interactions were observed between protein level and raising system for some parameters studied (relative weights of gizzard crest and dewlap and for serum protein, uric acid, and total cholesterol content). The relative gizzard weight of birds reared in cage system was not affected by CP but as the dietetic CP content increased in birds reared in floor system there was a decrease of relative gizzard weight. The gizzard plays an important function in digestive process of diets, so according to Svihus et al [34] there are a renewed interest in nutritional effects in diets that can stimulate the development and function of the gizzard. Freitas et al [28] concluded that the neutral detergent fiber reduced gizzard weight of hens. In the present study, the reduction of CP was beneficial to gizzard development, however, more studies are required to elucidate these findings.
The reduction of CP until 140 g/kg CP and 150 g/kg to laying hens reared in cage and floor system, respectively, resulted in the highest relative crest weight. Birds on cage system showed greater relative dewlap weight when fed with 140 and 160 g/kg CP. Birds on floor system showed greater relative dewlap weight when fed diets with 160 and 180 g/kg of CP. These sexual characteristics of laying hens could be associated with productive performance and requires further investigation.
The diets with 140 g/kg CP resulted in lower serum protein and lower total cholesterol content in birds reared in floor system, indicating a possible CP deficiency. In contrast, birds reared in cage system showed no effect of CP on serum protein and total cholesterol. Some factors are related to cholesterol metabolism in hen, as different levels of methionine throughout the day [35] and fiber intake [36], however the hypocholesterolemic effect associated to the protein is unclear.
Notes
IMPLICATIONS
This study demonstrate that the dietary protein level can be reduced up to 140 g/kg for Hisex Brown hens from 30 to 45 weeks of age, in both rearing systems, cage and floor.
CONFLICT OF INTEREST
We certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
FUNDING
The authors would like to thank the Instituto Federal Goiano/Campus Urutaí, and the Olvego Óleos Vegetais Ltda, MCassab and Ajinomoto® for financial support for this study.