Alfnes F, Guttormsen AG, Steine G, Kolstad K. 2006. Consumers’ willingness to pay for the colors of salmon: a choice experiment with real economic incentives. Am J Agri Econ 88:1050–1061.

Allen SK. 1998. Commercial applications of bivalve genetics: not a solo effort. World Aquaculture 29:38–43.
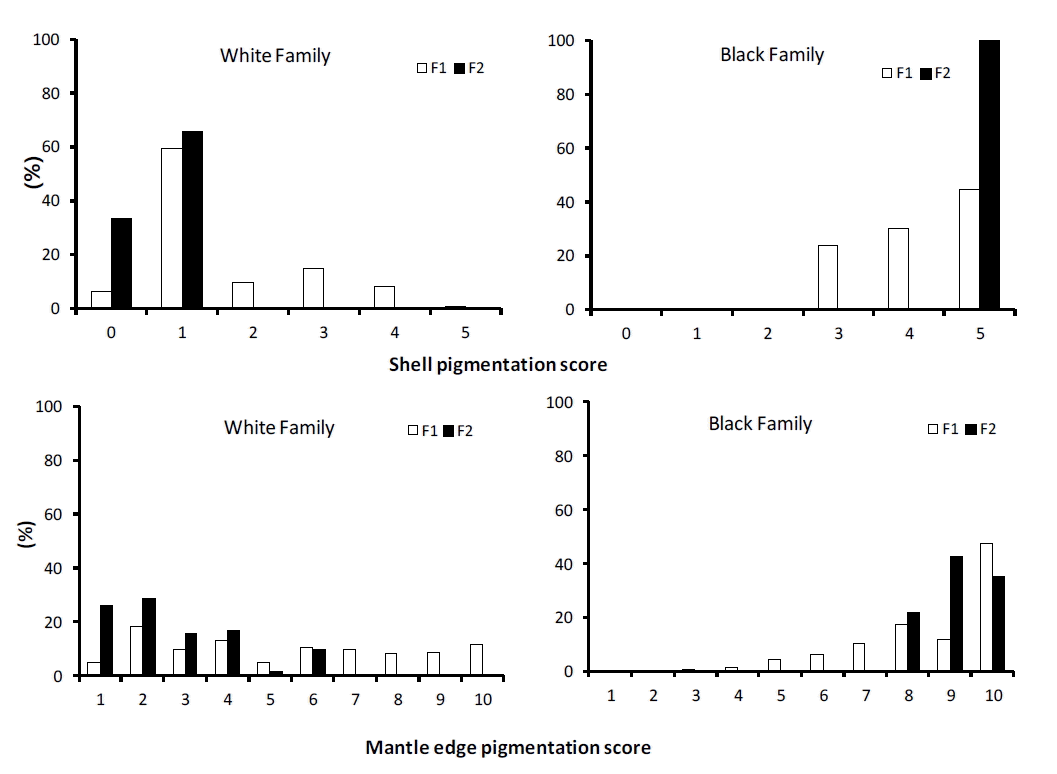
Brake J, Evans F, Langdon C. 2004. Evidence for genetic control of pigmentation of shell and mantle edge in selected families of Pacific oysters,
Crassostrea gigas. Aquaculture 229:89–98.

Clydesdale FM. 1993. Color as a factor in food choice. Crit Rev Food Sci Nutr 33:83–101.


Evans S, Camara MD, Langdon CJ. 2009. Heritability of shell pigmentation in the Pacific oyster,
Crassostrea gigas. Aquaculcure 286:211–216.

Gjerde B, Gjedrem T. 1984. Estimates of phenotypic and genetic parameters for carcass traits in Atlantic salmon and rainbow trout. Aquaculture 36:97–110.

Gjerde B, Schaeffer LR. 1989. Body traits in rainbow trout: II. Estimates of heritabilities and of phenotypic and genetic correlations. Aquaculture 80:25–44.

Gomelsky B. 2011. Fish genetics: theory and practice. VDM Verlag Dr. Mueller; Saarbrücken, Germany: p. 200
Gomelsky B, Cherfas NB, Ben-Dom N, Hulata G. 1996. Color inheritance in ornamental (koi) carp (Cypprinus carpio L.) inferred from color variability in normal and gynogentic progenies. Isr J Aquac-Bamidgeh 48:219–230.
Guo X, Allen SK. 1994. Viable tetraploids in the Pacific oyster (Crassostrea gigas Thunberg) produced by inhibiting polar body I in eggs from triploids. Mol Mar Biol Biotechnol 3:42–50.
Haskin HH, Ford SE. 1979. Development of resistance to Minchinia nelsoni (MSX) mortality in laboratory-reared and native oyster stocks in Delaware Bay. Marine Fisheries Review 41:54–63.
Hedgecock D, Grupe P. 2006. Mapping genes affecting shell color and shape in the Pacific oyster, Crassostrea gigas. J Shellfish Res 25:738
Imai T, Sakaki S. 1961. Study of breeding of Japanese oyster, Crassostrea gigas. Tohoku J Agric Res 12:125–171.
Lutz GC. 2001. Practical genetics for aquaculture. Fishing News Books; Oxford, UK: p. 235
MIFAFF. 2012. Ministry for Food, Agriculture, Forestry and Fisheries of Korea. Fisheries information service, Annual Statistics of Fisheries Production. (
http://www.fips.go.kr) Accessed January 8, 2013.
Naciri-Graven Y, Martin AG, Baud JP, Renault T, Gerard A. 1998. Selecting the flat oyster
Ostrea edulis (L.) for survival when infected with the parasite
Bonamia ostreae. J Exp Mar Biol Ecol 224:91–107.

Nell JA. 2001. The history of oyster farming in Australia. Mar Fish Rev 63:14–25.
Nell JA, Perkins B. 2006. Evaluation of the progeny of third-generation Sydneyrock oyster
Saccostrea glomerata (Gould, 1850) breeding lines for resistance to QX disease
Marteilia sydneyi and winter mortality
Bonamia roughleyi. Aquac Res 37:693–700.

Steine G, Alfnes F, Rørå MB. 2005. The effect of color on consumer WTP for farmed salmon. Mar Resour Econ 20:211–219.

Thorgaard GH, Spurell P, Wheeler PA. 1995. Incidence of albinos as a monitor for induced triploidy in rainbow trout. Aquaculture 137:121–130.

Ward RD, Englisj LJ, McGoldrick DJ, Maguire GB, Nell JA, Thompson PA. 2000. Genetic improvement of the Pacific oyster
Crassostrea gigas (Thunberg) in Australia. Aquac Res 31:35–44.

Withler RE, Beacham TD. 1994. Genetic variation in body weight and flesh colour of the coho salmon (
Oncorhynchus kisutch) in British Columbia. Aquaculture 119:135–148.






 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print







