INTRODUCTION
Soybean is a legume crop and a premier protein source of foods and feeds globally, owing to its well-balanced amino acid pattern (Thippeswamy and Mulimani, 2002; Stein et al., 2008). Nevertheless, the use of soybean-based foods or feeds has been occasionally restricted because they contain α-galacto-oligosaccharides such as raffinose and stachyose, which act as anti-nutritive factors (De Lumen, 1992; Anderson and Wolf, 1995; Prashanth and Mulimani, 2005). Monogastric animals including human, swine, and poultry cannot synthesize sufficient α-galactosidase (EC 3.2.1.22) in their gastrointestinal tracts (Karr-Lilienthal et al., 2005; Prashanth and Mulimani, 2005), which is an exo-glycosidase catalyzing the hydrolysis of terminal non-reducing α-1,6-linked galactosyl residues from a wide range of galacto-oligosaccharides and polysaccharides (Naumoff, 2004). Thus, the α-galacto-oligosaccharides pass into the large intestine, where the resident microbita ferment them into carbon dioxide, hydrogen, and methane, which lead to flatulence and gastrointestinal disorder (Steggerda et al., 1966; Prashanth and Mulimani, 2005). Accordingly, degradation of the oligosaccharides from the soybean-based products by α-galactosidase could be an efficacious tool to solve the nutritional problem (Ghazi et al., 2003; Gote et al., 2004; Falkoski et al., 2009).
Considerable attention has been paid to immobilized enzymes, because of their favorable features such as reusability, stability, and separation of the enzymes from products, compared to free counterparts (Falkoski et al., 2009). Particularly, concerning animal nutrition and feed science, the immobilization technique has been applied in developing a kit for measuring in vitro amino acid digestibility of soybean meal, which could be correlated to poultry true amino acid digestibility (Schasteen et al., 2007) and in preparing a forage additive (Monica et al., 2008). Up to now, several matrices including polyacrylamide, alginate, chitin, silica gel, Amberlite IRA-938, and Sepabeads EC have been used as support materials for preparing immobilized α-galactosidases (Onal and Telefoncu, 2003a,b; Prashanth and Mulimani, 2005; Falkoski et al., 2009; Bayraktar et al., 2011). Additionally, there is much interest in Eudragit L-100 (a copolymer of methacrylic acid and methylmethacrylate) which was utilized as a reversibly soluble and insoluble immobilization matrix for immobilization of the widely used technological enzymes such as xylanase (Sardar et al., 2000; Gaur et al., 2005), cellulase (Zhang et al., 2010), and amylase (Cong et al., 1995), as well as a pH-dependent enteric coating polymer for the efficacious colon-specific drug delivery system (Venkatesh et al., 2009).
To the best of our knowledge, the present study is the first report on the immobilization of α-galactosidase on Eudragit L-100. The α-galactosidase was derived from an Antarctic bacterial isolate, Bacillus LX-1 as previously described (Lee et al., 2012).
MATERIALS AND METHODS
Reagents
Eudragit L-100, which is a copolymer of methacrylic acid and methylmethacrylate at a ratio of 1:1, was a product of Rohm Pharma, Weiterstadt, Germany. The substrate, p-nitrophenyl-α-D-galactopyranoside (pNPG) was purchased from Sigma-Aldrich (St. Louis, MO, USA). All other chemicals used were of analytical grade.
Preparation of free α-galactosidase
Bacillus sp. LX-1 was cultivated in 25 ml of Luria-Bertani (LB) medium supplemented by containing 1% galactose in an Erlenmeyer flask of 250 ml capacity for 24 h at 28°C. Then, 1 L of the same medium in two Erlenmeyer flasks of 2 L capacity was aseptically inoculated with 1% seed culture broth and aerobically grown with vigorous shaking (220 rpm) for 48 h at 28°C. The culture medium containing secreted α-galactosidase was centrifuged (10,000×g; 20 min; 4°C) to remove cell, and then protein in the supernatant was precipitated with ammonium sulfate (75% saturation). The pellet was dissolved in 25 mM Tris-HCl (pH 8.0) and dialyzed overnight against 25 mM Tris-HCl (pH 7.4) at 4°C. The dialyzed solution was used as the free enzyme throughout this work.
Immobilization of α-galactosidase on Eudragit L-100
2% Eudragit L-100 solution was prepared as previously described (Roy et al., 2003). The free enzyme (0.1 to 2 ml) was added to 0.75 ml of the Eudragit L-100 solution and the final volume was made up to 5 ml with 50 mM sodium phosphate (pH 7.0). After 1 h incubation at room temperature, polymer was precipitated by lowering pH to 4.0 with 3 M acetic acid. After 20 min, the suspension was spun down by centrifugation (12,000×g, 20 min). The precipitate was washed with 4 ml of 10 mM sodium acetate (pH 4.0) until no enzyme activity was detected in the washings. Finally, the pellet so formed was suspended in 5 ml of 50 mM sodium phosphate (pH 7.0) and used as the immobilized enzyme preparation.
Enzyme activity assay
The α-galactosidase activity was determined by the amount of p-nitrophenol released from the pNPG. The reaction mixture (1 ml) containing 0.1 ml of diluted soluble enzyme (free or immobilized), 0.8 ml of 50 mM sodium phosphate (pH 7.0 ) and 0.1 ml of 10 mM pNPG in 50 mM sodium phosphate (pH 7.0) was incubated at 40°C for 15 min. The reaction was terminated by adding 1 ml of 1 M Na2CO3. The absorbance was measured at 405 nm. One unit (U) of enzyme activity was defined as the amount of enzyme required to liberate 1 μmol of p-nitrophenol per minute under the assay conditions.
Reusability of the immobilized enzyme
The immobilized α-galactosidase was used for repeated cycles to check its reusability. The preparation was incubated at 40°C with 1 mM pNPG in 50 mM sodium phosphate (pH 7.0) for 15 min. After each cycle of hydrolysis, the pH of the supernatant was lowered to 4.0 by adding 0.2 ml of 3 M acetic acid when the immobilized enzyme was precipitated. This was collected by centrifugation (12,000×g, 30 min) and the amount of released p-nitrophenol in supernatant estimated according to the analytical method described above. For running the second cycle, the immobilized enzyme was redissolved in 5 ml of 50 mM sodium phosphate (pH 7.0) and added to the pNPG and processed the same way as before.
Determination of pH optimum
The effects of optimum pH on the free and immobilized LX-1 α-galactosidase were investigated by assaying both preparations in the pH range of 3 to 8.5 (50 mM glycine-HCl (pH 3); 50 mM sodium acetate (pH 4 to 5.5); 50 mM sodium phosphate (pH 6 to 7) and 50 mM Tris-HCl (pH 7.4 to 8.5)) at 30°C.
RESULTS AND DISCUSSION
Immobilization of LX-1 α-galactosidase
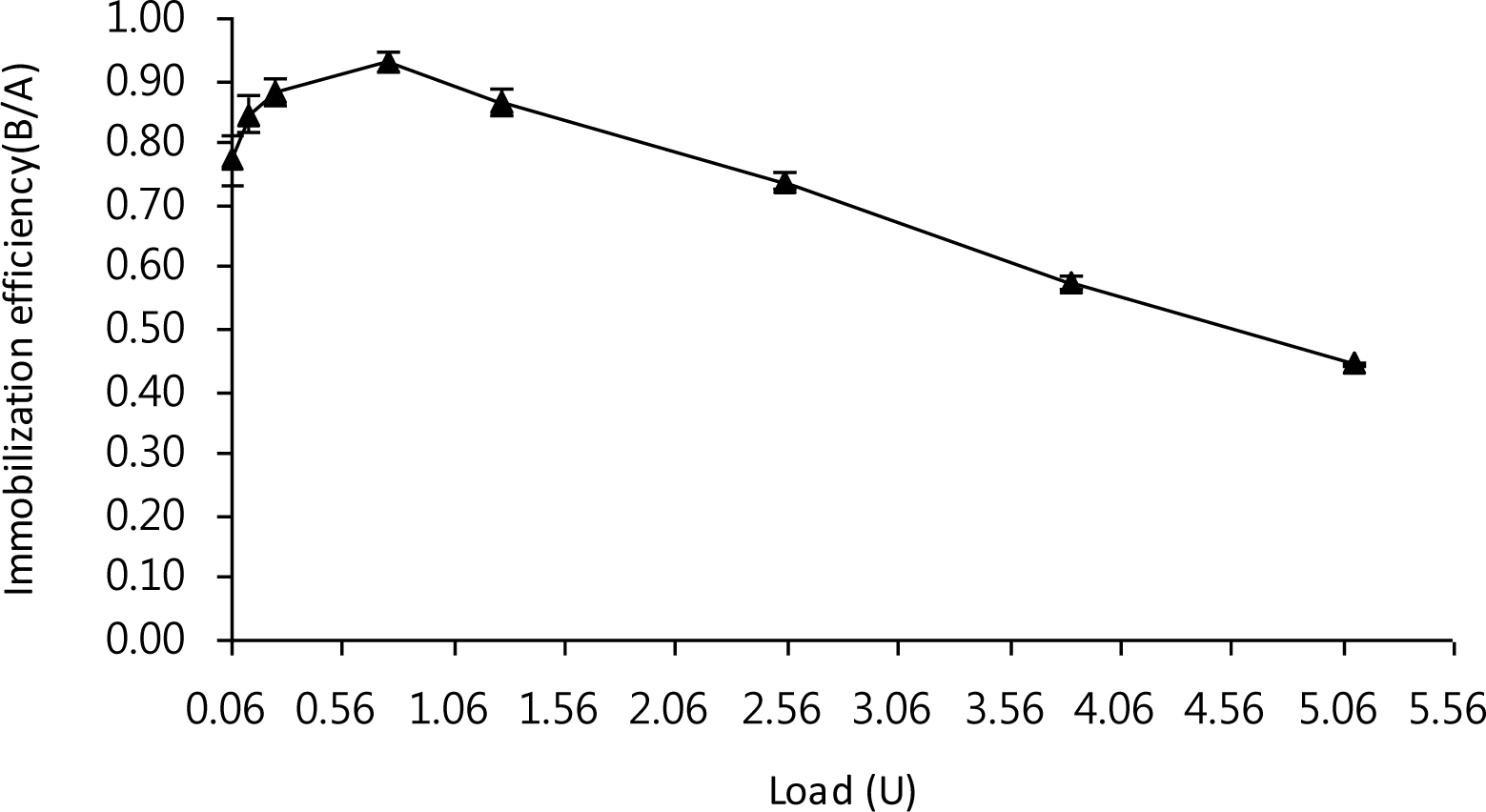
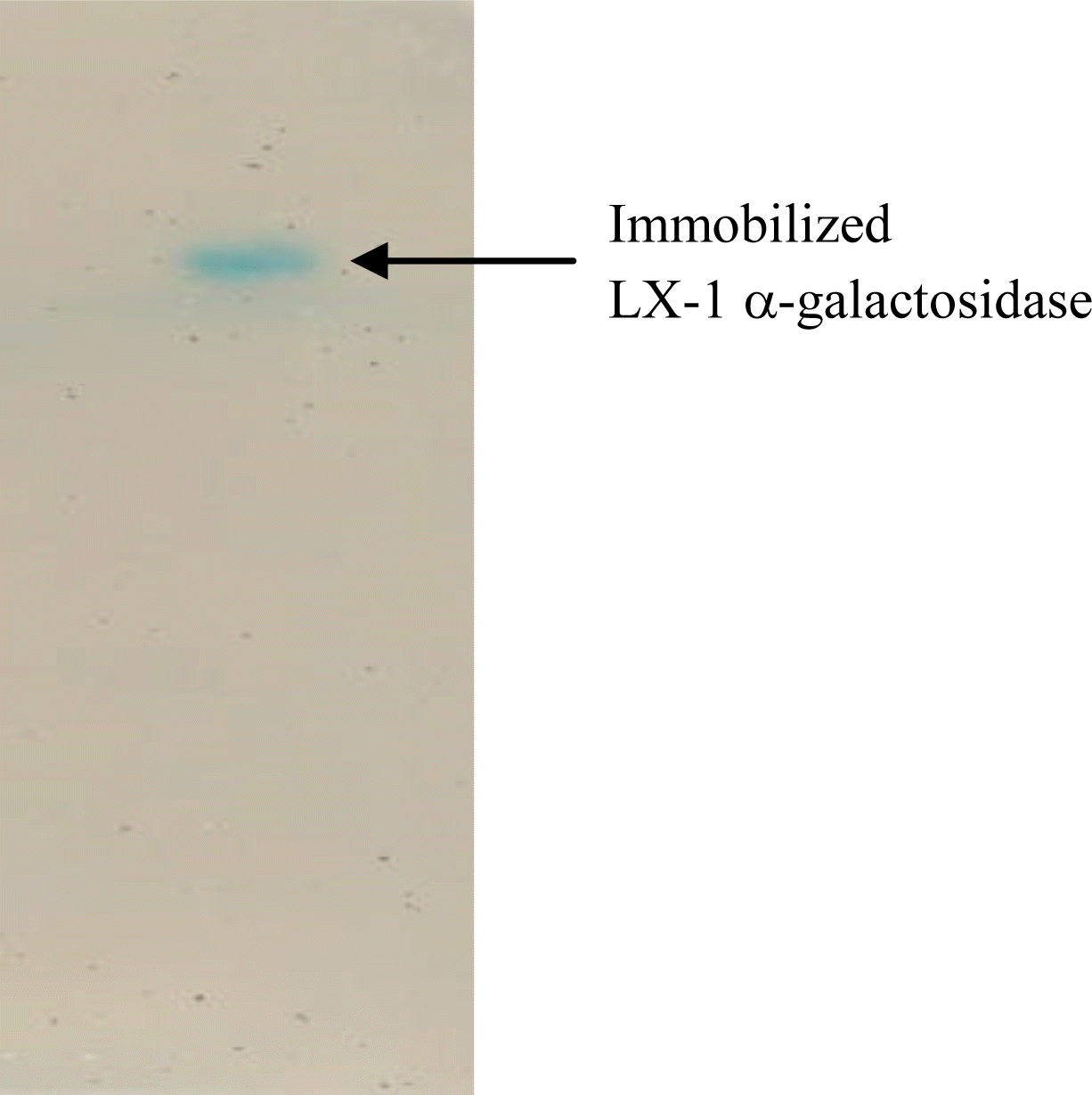
LX-1 α-galactosidase was immobilized on the smart polymer, Eudragit L-100, which is a relatively cheap, easily available, non-toxic, and water soluble support, and which binds non-covalently to proteins (Gaur et al., 2005). The optimal immobilization efficiency of 0.93 was achieved at 0.77 U of enzyme load and, beyond the amount of enzyme, the immobilization efficiency tended to decrease (Figure 1). Figure 2 depicted the activity staining of the immobilized enzyme. The decrease of immobilization efficiency with larger enzyme load is generally caused by overcrowding of the enzyme on the Eudragit surface (Sardar et al., 2000; Roy et al., 2003). Meanwhile, only 0.55 of the optimal immobilization efficiency was observed when α-galactosidase from Penicillium griseoroseum was immobilized onto modified silica gel using glutaldehyde linkages (Falkoski et al., 2009). Thus, the immobilization yield of LX-1 α-galactosidase seemed to be acceptable.
Reusability of immobilized enzyme
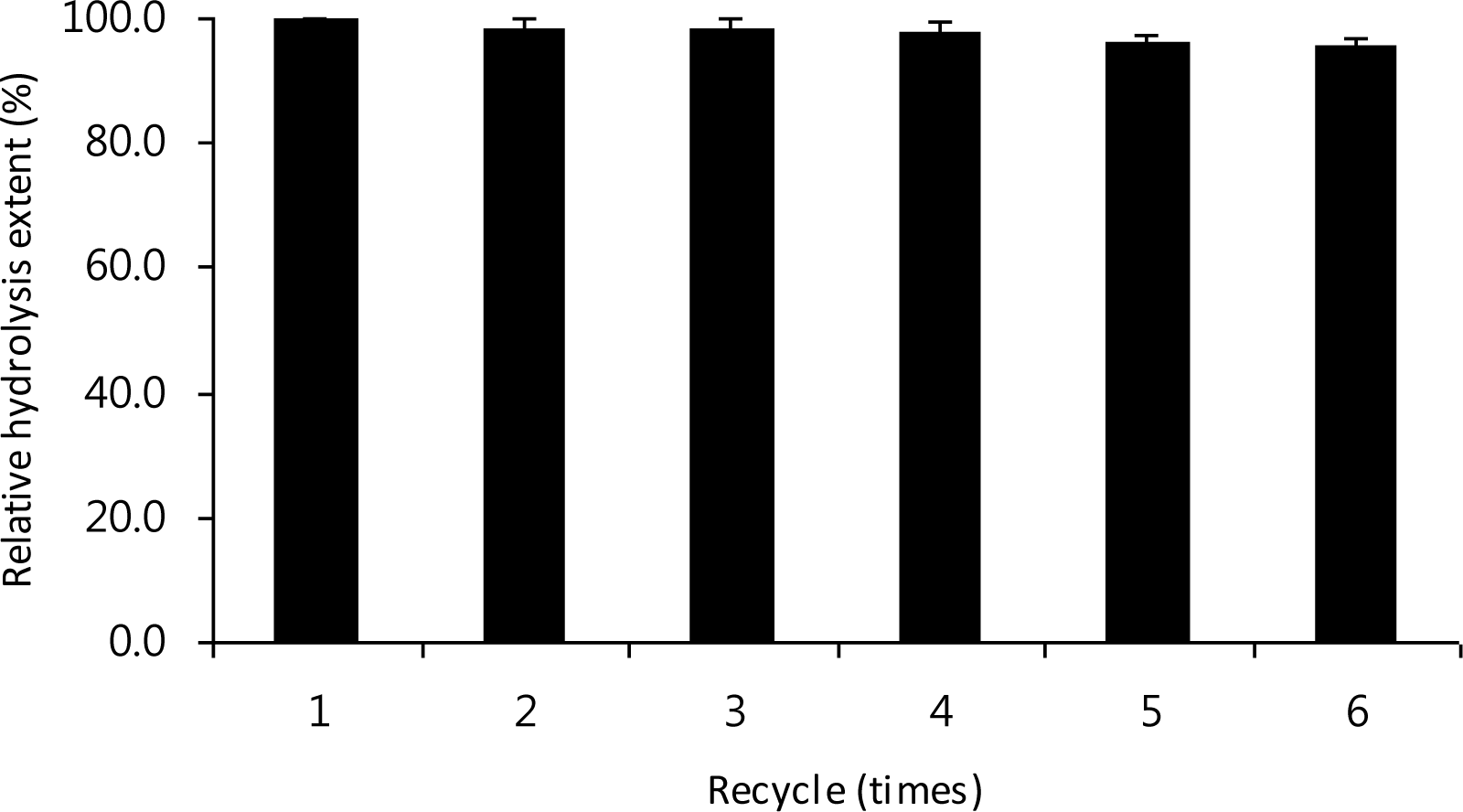
As an immobilization matrix, the merit of Eudragit L-100 is its solubility during catalysis with its insolubility below pH 4.0, enabling catalyst recovery and separation of the soluble reaction products by lowering the pH (Roy et al., 2003; Gaur et al., 2005). Reusability of an immobilized enzyme is of great importance for economical use of the enzyme in repeated batch or continuous processes (Bayraktar et al., 2011). As shown in Figure 3, the immobilized LX-1 α-galactosidase could be recycled for six times without significant loss in activity, despite the fact that the enzyme was recovered by lowering the pH to 4.0 after each cycle. Such acidic process for enzyme recovery, which has routinely used in other enzymes immobilized on Eudragit matrices (Gaur et al., 2005; Silva et al., 2006; Zhang et al., 2010) did not have any negative effect on the LX-1 α-galactosidase activity. This may be somewhat associated with previous report that immobilization of Aspergillus niger xylanase on Eudragit L-100 has led to stabilization of the enzyme toward alternate exposure to pH 4.0 and pH 5.5 (Sardar et al., 2000).
Effect of pH on activity of free and immobilized enzyme
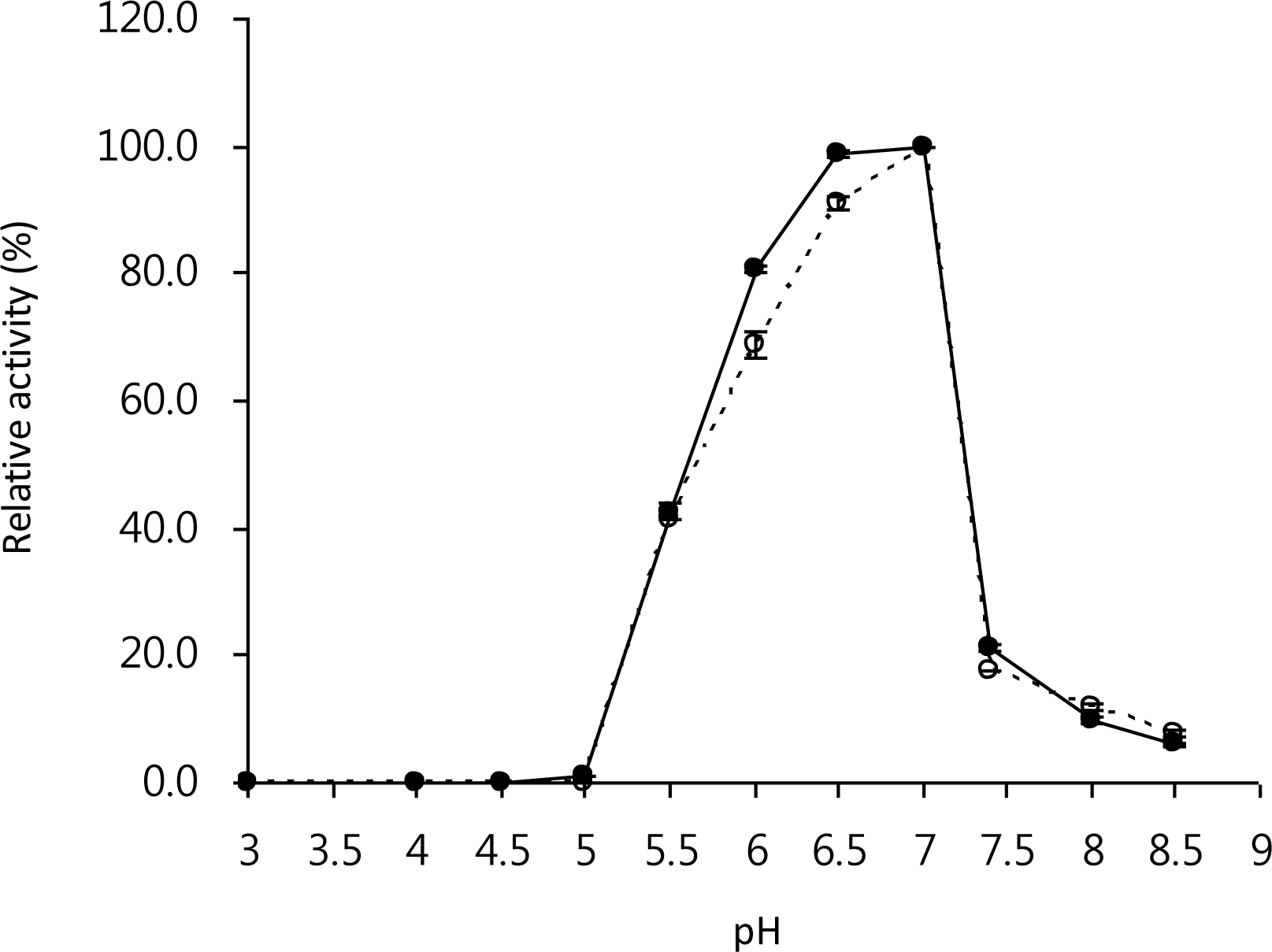
As shown in Figure 4, the optimum pH values for the free and immobilized LX-1 α-galactosidase were 6.5 to 7.0 and 7.0, respectively. Although the pH profile change in immobilized enzymes was very common (Thippeswamy and Mulimani, 2002; Bora et al., 2005; Sanjay and Sugunan, 2005), immobilization of enzymes on the reversibly soluble-insoluble polymers such as Eudragit L-100 and Eudragit S-100 had frequently marginal effect on the optimal pH activities (Ai et al., 2005). For instance, there were no and slight changes in the optimum pH values for Aspergillus niger and Scytalidium thermophilum xylanase immobilized on Eudragit L-100, respectively (Sardar et al., 2000; Gaur et al., 2005). In addition, the optimum pH value of tomato α-galactosidase immobilized on galactose-containing polymeric beads and the free enzyme was identically pH 4.0 (Okutucu et al., 2010).
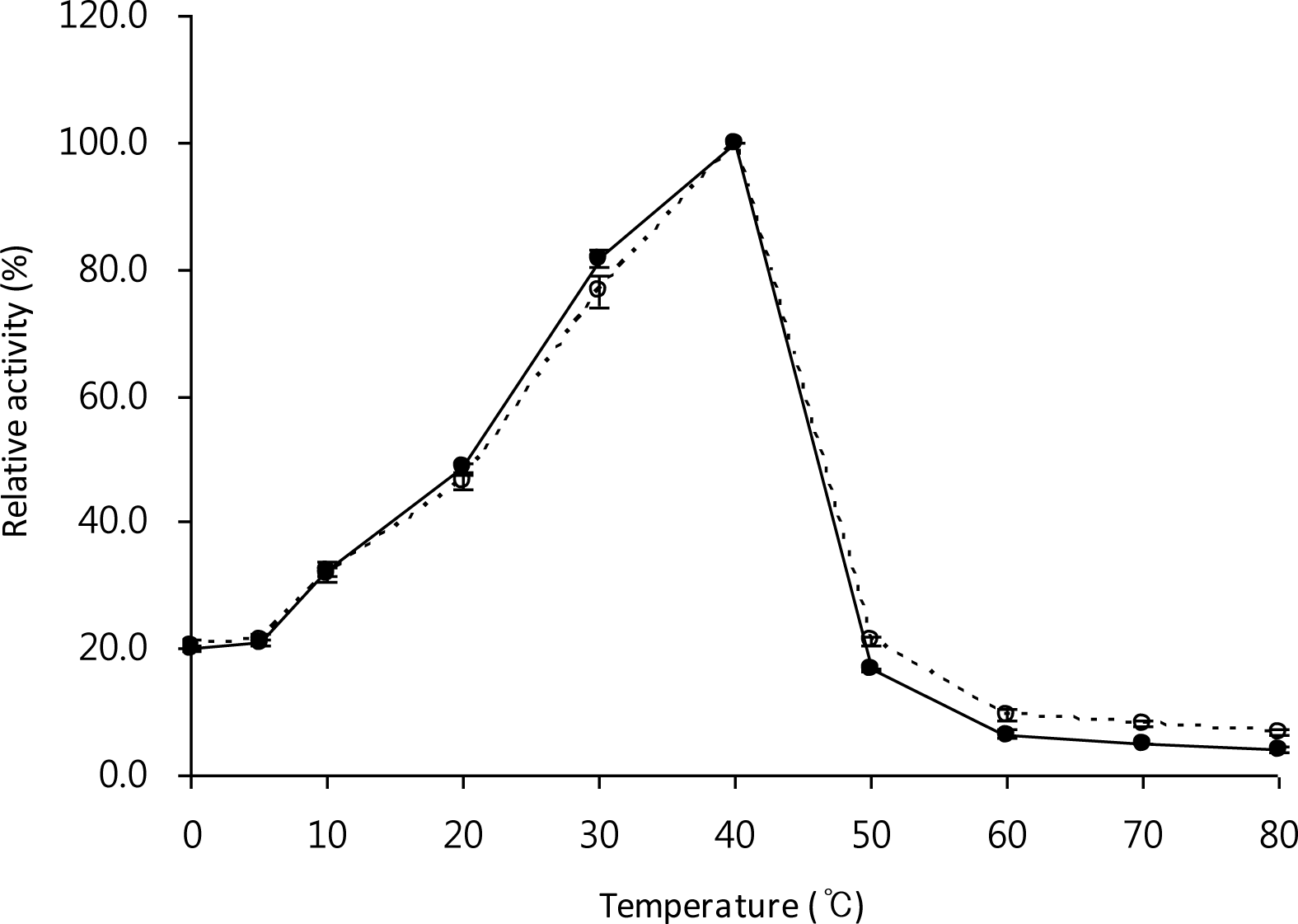
Effect of temperature on activity of free and immobilized enzyme
The optimum temperature for the free and immobilized LX-1 α-galactosidase was 40°C (Figure 5). Immobilization had no influence on the optimum temperature of the enzyme, which was also observed in a commercial protease, Esperase, immobilized on Eudragit S-100 (Silva et al., 2006), the Penicillium griseoroseum α-galactosidase immobilized on silica gel (Falkoski et al., 2009) and the tomato α-galactosidase immobilized on galactose-containing polymeric beads (Okutucu et al., 2010). However, the optimum temperature of the Streptomyces olivaceoviridis E-86 xylanase immobilized on Eudragit S-100 moved from 60°C (the optimum temperature of free enzyme) to 65°C (Ai et al., 2005).
Storage shelf stability of free and immobilized enzymes
In general, an enzyme in solution is not stable during storage and the activity is gradually decreased over time (Bayraktar et al., 2011). From a practical point of view, it is an important criterion that an enzyme designed for use in animal feed should survive storage at ambient temperature (Francesch and Perez-Vendrell, 1997; Sulabo et al., 2011). As shown in Figure 6, the storage stability at 37°C was greatly improved by immobilization. The immobilized LX-1 α-galactosidase retained about 50% of its initial activity even after 18 d at this temperature, while the free enzyme was completely inactivated. The improved stability of immobilized enzymes may be associated with the prevention of autolysis (Sharma et al., 2003). In addition, the storage stability of immobilized enzymes varies depending on the applied immobilization method and storage conditions (Onal and Telefoncu, 2003b; Celem and Onal, 2009; Okutucu et al., 2010).
CONCLUSIONS
Nowadays, advanced feed production can rely on biotechnological approaches. The immobilization of α-galactosidase from Bacillus sp. LX-1 on Eudragit L-100 may be a promising strategy for removal of α-galacto-oligosaccharides such as raffinose and stachyose from soybean meal and other legume in feed industry, which enables to increase the available energy and feed efficiency (Suarez et al., 1999; Vila and Mascarell, 1999), due to its relatively durable enzyme activity and good storage stability.






 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print







