Effect of Cattle Breeds on Milk Composition and Technological Characteristics in China
Article information
Abstract
Cattle breeds have a striking effect on milk, including milk composition and technological characteristics. This study aims to compare milk composition, acidification activity, viscosity, milk dispersion system stability and casein molecular weight among three buffalo breeds in China. The technological characteristics of milk produced by three cattle breeds of river buffalo (Murrah), crossbreed 1st generation (F1), crossbreed multiple generation (FH, H≥3) buffaloes were investigated. Cattle breeds showed evident effect on milk protein, fat and total solids content, but little effect on most of buffalo casein molecular weight. Milk fat, protein content and the viscosity of buffalo milk from river buffalo were lower than those of F1 and FH, so was the buffer capacity. The viscosity was negatively correlated to temperature and concentration. Results of stability coefficient showed that milk dispersion system had the best dynamic stability characteristics under pH 6.6 and 6 times dilution, while zeta potential of Murrah milk was slightly higher than that of hybrid offspring (F1, FH). SDS-PAGE results showed that buffalo αs-casein had a slightly faster mobility than standard αs-casein; while buffalo β-casein showed a slightly slower mobility than standard β-casein. There is no clear differences in molecular weight of αs-, β-, and κ-casein among Murrah, F1 and FH.
INTRODUCTION
Cattle breeds have a striking effect on meat and fat color, muscle structure and meat physiology (Waritthitham et al., 2010; Xie et al., 2012), as well as their product (milk). Because of genetic background, milk from different cattle breeds holds distinct composition profiles (Poulsen et al., 2012). The nutritional value of buffalo milk was higher than that of Holstein cow milk (Lindmark-Månsson et al., 2003; Benincasa et al., 2008; Maurice-Van Eijndhoven et al., 2011). Buffalo breeds contain swamp buffaloes and river buffaloes, which accounted for 67% and 33% respectively. Swamp buffaloes (karyotype 2n = 48) are draft animals and have low performance in milk production (milk yield, ∼700 kg per cow per annum), but high total solids (Ts) content; river buffaloes (karyotype 2n = 50), as represented by Murrah buffalo, have a good milk yield (∼2,200 kg per cow per annum), but lower Ts content compared with swamp buffaloes (Han and Ding, 1994). Most of the swamp buffaloes are distributed throughout south China. In order to improve the quality and yield of local buffalo (swamp buffalo) milk, the river buffalo (♂) was hybridized with the local swamp buffalo (♀). The hybrid offspring buffaloes produce milk with a higher content of dry matter compared with the milk from river buffalo (Han et al., 2007). In recent years, buffaloes have been rapidly developed as milk producer, and the yield of hybrid buffalo milk increases substantially in China (Pang et al., 2007).
The technological characteristics of milk closely relate to the texture and stability of dairy products based on the milk composition. They are affected by temperature (Jeurnink and Kruif, 1993), concentration, and pH value among other factors (Ahmad et al., 2008; Ménard et al., 2010). Although a great deal of work has been done on the physic-chemical properties of cow milk, even on some of the physic-chemical properties of buffalo (Bubalus bubalis) milk (Jeurnink and Kruif, 1993; Anema and Klostermeyer, 1996; Ahmad et al., 2008; Ménard et al., 2010), and the effects of feeding and management of cattle on milk composition have also been explored by a number of researchers (Maurice-Van Eijndhoven et al., 2011; Norrapoke et al., 2012; Terramoccia et al., 2012), little is known about the technological characteristics of milk from different buffalo breeds. Moreover, the technological properties and chemical composition of crossbreed buffalo milk remains unclear.
Because of the known general milk composition (Han and Ding, 1994) and the lower milk yield, the technological properties of local swamp buffalo will not be considered in this work. Therefore, in order to get more information about the technological properties of milk from different buffalo breeds to produce the suitable dairy products, this study was carried out to evaluate the technological properties and the main differences in composition among three local buffalo breeds in China, which were Murrah buffalo (river buffalo), crossbreed offspring (river buffalo♂×swamp buffalo♀) 1st generation buffalo (F1), and crossbreed multiple generation buffalo (FH, H≥3) that was produced by breeding with river buffalo as shown in Figure 1.
MATERIALS AND METHODS
Milk samples
Fresh whole milk samples from Murrah (river buffalo), crossbreed (river buffalo♂×swamp buffalo♀) 1st generation (F1), and crossbreed multiple generation (FH) buffaloes were collected in Wutang farm (indoors) of Nanning, Guangxi, China. Total of 108 milk samples (36 from each breed) were collected. 108 buffaloes were fed a total mixed rations (TMR) with a forage/concentrate ratio of 70/30. The diet consisted of grassiness (35%), maize stalk silage (35%), and concentrate (30%) made from maize (45%), soybean pomace (20%), wheat bran (15%), cottonseed meal (10%), by-products such as brewers dried grain (5 kg per cow per day), and salt and mineral elements (10%). The diet was provided by Dr. Wei from Wutang farm, Nanning, Guangxi, China. Management practices were applied equally to all buffaloes. Standards of α-casein (α-CN), β-casein (β-CN), and κ-casein (κ-CN) were from Sigma (Sigma-Aldrich [Shanghai] Trading Co., Ltd. Shanghai, China). Holstein cow casein provided by our laboratory was used as a reference. Molecular weight marker 10 to 200 kDa (#SM0661) was obtained from Fermentas (USA). Other reagents were all of analytical grade.
General composition
Milk fat and crude protein content were determined by the Babcock and Kjeldahl methods, respectively. Lactose was determined by colorimetric method according to the Chinese standard method (GB/T 16285-1996). Ts were determined by conventional ovendrying (60°C for 2 to 3 h, then 100°C for 6 h) of 5 g milk sample. The pH was measured using a Microcomputer pH meter (Sartorius Instruments, Germany); calibration was done with buffers of pH 4.00 and pH 6.80.
Buffer capacity
The buffering capacity was measured according to Van Slyke with some modifications (Slyke, 1922). Briefly, 100 ml milk were slowly added with 0.1 M HCl or 0.1 M NaOH under vigorous stirring at room temperature (25±1°C). All the experiments were carried out in duplicate.
Stability coefficient
One ml of each milk sample was 1:20 diluted with Milli Q-water at room temperature and divided into two parts. For one part, the pH values were adjusted from pH 4.2 to pH 7.0 with 0.2 M phosphate buffer, the other part was diluted 2 to 8 times again, and proper amount of samples was centrifuged at 1,420 g for 5 min. The absorbance of all samples was measured at 780 nm using an ultraviolet spectrophotometer (1601 ultraviolet spectrophotometer, Shimadzu).
Viscosity measurements
Viscosity measurements were performed using an Ostwald capillary viscometer (Shanghai Liangjing, China) by the method of Jeurnink and de Kruif (Jeurnink and Kruif, 1993) with slight modification. One part of sample was measured at various temperatures from 4 to 95°C, the other part was diluted 2 to 8 times and measured at room temperature. Samples were equilibrated in a temperature-controlled thermostat water bath for 30 min before viscosity measurements at each temperature.
Zeta potential measurements
Zeta potentials (the net charge on a particle, in mV) were measured by laser Doppler electrophoresis with the Nano-S Zetasizer (Malvern Instruments Limited, Malvern, Worcestershire, UK). Samples were measured under various pH (from 4.5 to 7.0) and dilution ratio (all milk samples were diluted 2, 4, 6, 8 times again with Milli Q-water) after dilution for 200 times with Milli Q-water as described previously (Liu et al., 2011).
Gel electrophoresis analysis
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) of the samples was carried out on a Bia-Rad System (Mini-Protean 3 cell, USA) as described previously (Laemmli, 1970). The protein samples were solubilized with phosphate buffer after ultracentrifugation at appropriate concentrations at 20°C. Gel was stained with Coomassie blue dye for 2 h, followed by destaining in a solution containing 30% methanol and 10% acetic acid.
Statistical analysis
All data were analyzed by one-way ANOVA using the statistical analysis software (SPSS Statistics 17.0, 2010). Differences with p<0.05 were considered statistically significant.
RESULTS AND DISCUSSION
General composition
The general composition of three types of milk were significantly different (p<0.05) among the three cattle breeds under the same feeding condition (Table 1). Ts content of swamp buffalo (22.64%, w/w; Han and Ding, 1994) was higher than that of river buffalo (17.23%, w/w) and their hybrid offspring (19.05%, w/w). The milk yield of local swamp buffalo was low (∼700 kg per cow per annum), but the Ts content was high; on the contrary, Murrah showed the high milk yield (∼2,200 kg per cow per annum) and low Ts content. In the current study, it is important to note that the milk yield of crossbreed offspring (F1, ∼1,500 kg per cow per annum; FH, ∼2,100 kg per cow per annum) was higher than that of the swamp buffalo (female parent), and the milk compositions including fat, protein and Ts contents of crossbreed offspring (F1 and FH) were higher than those of Murrah (male parent), and all above composition contents of F1 were higher than those of FH. There was no great difference between crossbreed offspring milks, but F1 milk was 5.71% higher than the other buffalo milk, indicating that F1 buffalo milk had the highest nutritive value. Fat, protein, and Ts contents of FH milk were 22.71%, 6.67%, and 7.84% respectively higher than those of Murrah. This also inferred that the more the multi-generations, the closer the main milk composition to their male parent’s.
Buffer capacity of three cattle breeds
The acidification of milk from different buffalo breeds was studied by ANOVA. Nevertheless, the buffer capacity of the river buffalo milk was higher than that of the hybrid offspring buffalo milk (p<0.05), and the buffer capacity of F1 milk was slightly higher than that of FH milk (Figure 2). The milk protein contents of the three local breeds were ranked as follows: F1>FH>Murrah (Table 1). The different buffer capacities were probably related to the higher casein content in buffalo milk same as the buffer properties of milk. It demonstrated that there were no clear correlation between milk protein content and buffer capacity. The buffer capacity of milks might also relate to their composition of acido-basic compounds, calcium phosphate, and phosphorylation of casein (Salaün et al., 2005). Moreover, the protein content and molecular organization also plays an important role in buffering capacity, e.g., inorganic phosphate contributes to the buffering capacity too (Ahmad et al., 2008). These results indicated that F1 had a better buffer capacity with much more buffering components, such as higher contents of protein, Ts, as well as inorganic phosphate.
Effect of concentration and pH of three cattle breeds on milk stability coefficient
Stability is an important attribute of dairy products. The stability coefficient (R) of samples was determined under different pH and dilutions (Figures 3 and 4).

Stability coefficient of milk samples as a function of pH. R is the ratio of absorbance before and after samples centrifugated, which was determined by a ultraviolet spectrophotometer (UV-1601 ultraviolet spectrophotometer, Shimadzu), at 780 nm. •, Murrah; Δ, F1;▴, FH. The data presented are averages of three replications with the error bars indicating the standard deviation.
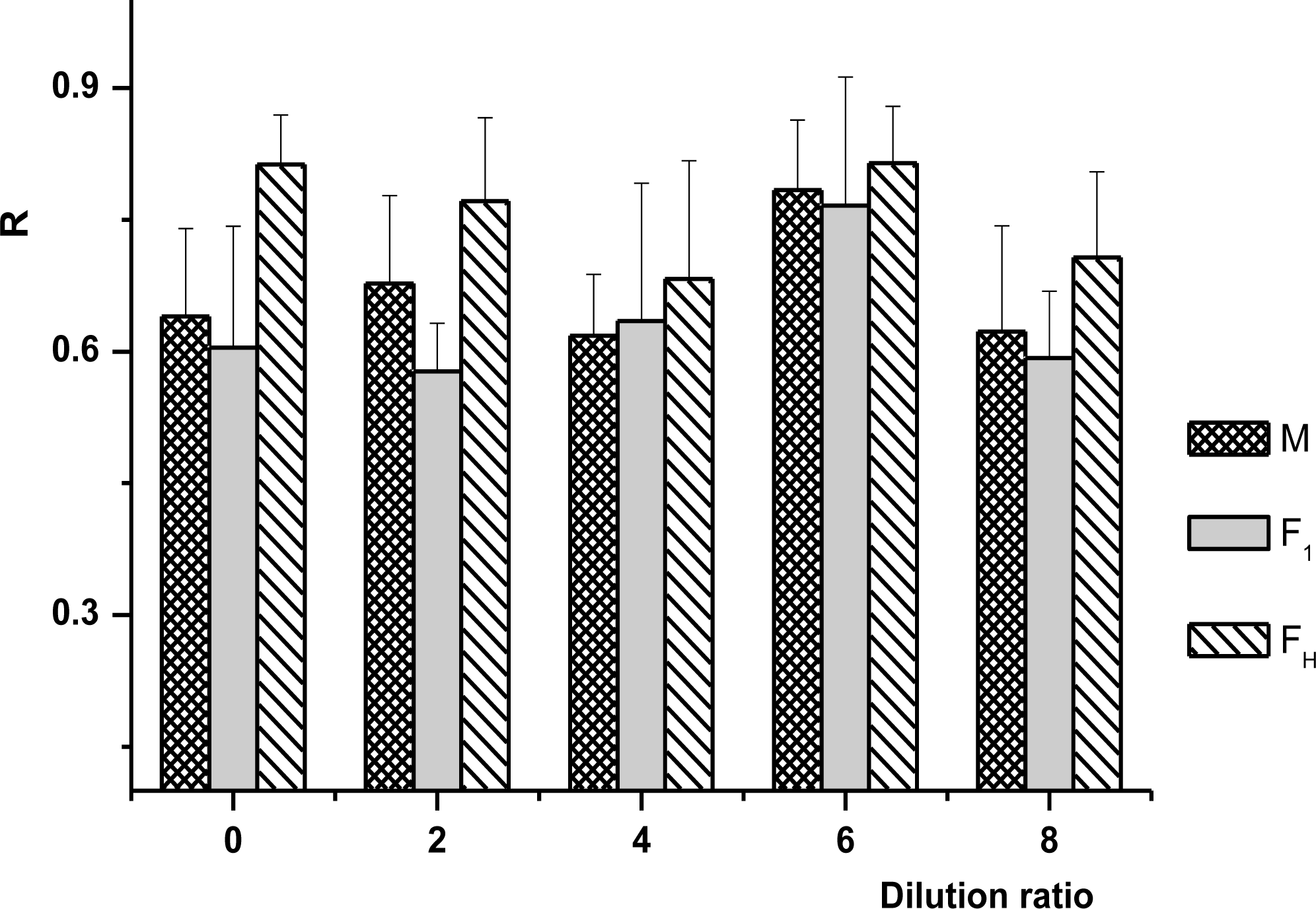
Stability coefficient of milk samples as a function of dilution ratio. R is the ratio of absorbance before and after samples centrifugated, which was determined by a ultraviolet spectrophotometer (UV-1601 ultraviolet spectrophotometer, Shimadzu), at 780 nm. M, Murrah. Results are the average of three repeated measurements with the error bars indicating the standard deviation.
R values of the three types of milk were high under natural pH ranging from 6.45 to 6.63. As shown in Figure 3, R values of all milk samples increased with the increasing pH value, and the maximum R value of Murrah and F1 milk appeared at pH 6.6; however, the R of FH reached maximum at pH 6.2 and was lower at pH 6.6, which was significantly different from that of other breeds. There were no evident differences in R values between Murrah and F1. These results indicated that the nuclear charge number of casein micelle of Murrah milk was similar to that of F1 near their natural pH value.
As the milk was acidified, the nuclear charge number of casein micelle changed gradually, and the hydration reduced slowly, but the interaction of sub-microsphere became weak. The aggregation of loosely entangled proteins was observed, which were presumably originated from proteins that dissociated from the casein micelles. These aggregates became larger with the decreased pH value and precipitated rapidly, particularly when the pH approached the isoelectric point of caseins. The R values of milk of all breeds decreased with the increasing pH from 4.2 to 5.0, and reached the minimum value at pH 5.0 except of that of Murrah milk, which was higher at pH 5.0 and reached the minimum at pH 5.4. The differences in minimum R values were not evident among all kinds of milk. It suggests that the hydrophobic interactions between proteins, casein dephosphorylation, and reassociation of proteins, destabilized the colloid system (McMahon and Oommen, 2008; McMahon et al., 2009; Kehoe and Foegeding, 2011), and all breeds of milk dispersion systems exhibited instability around pH 5.0. McMahon et al. (2009) reported the occurrence of acid gelation of milk at pH between 5.3 and 4.9, which involved a reassociation of loosely entangled protein aggregates into more-compact colloidal particles or association with any remaining casein supramolecules. However, this study found that the specific conditions for three types of milk were different.
R of milk varied as a function of dilution factor (Figure 4). It decreased with the increasing dilution ratio in the initial stage and then increased. The R values of Murrah, F1, and FH reached the peaks at 6 times diluted, suggesting that the dispersion system achieved a relatively stable state. There were no significant differences among all milk samples. Yet, the correlation between R and pH value was remarkable (p<0.05); after the correlation analysis by SPSS, the correlation coefficients between R and pH were 0.815, 0.772, and 0.751 for Murrah, FH, and F1 milk, respectively (p<0.05). However, the correlation between milk concentration and R was not significant (p>0.05, data not shown).
Effect of temperature and concentration of three cattle breeds on milk viscosity
There are few researches focused on the milk viscosity of different cattle breeds. Jeurnink and Kruif found that heating temperature, holding time, and β-lactoglobulin influenced the viscosity of bovine skim milk (Jeurnink ansd Kruif, 1993). Milk viscosities of three cattle breeds were presented in Figure 5 and 6. All milk viscosities were significantly (p<0.01) affected by temperature (Figure 5) and concentration (Figure 6). The correlation coefficients between viscosity and temperature analyzed by correlation analysis were −0.902, −0.858, and −0.854, and those between viscosity and concentration were −0.897, −0.894, and −0.877, for Murrah, FH, and F1, respectively. Figure 4 shows that milk viscosity decreased with the increasing temperature. The milk viscosity in all breeds sharply decreased from 4°C to 65°C. Meanwhile, it is easy to find that the viscosity of hybrid offspring milk was higher than that of river buffalo (Murrah) milk, and F1 was higher than FH (Figure 5). The average viscosity of hybrid offspring was 0.29 mPa.s, above that of Murrah milk. The viscosity slowly decreased from 60 to 95°C, and it showed no significant differences among all breeds (Figure 5). This was attributed to the denaturation of whey protein adhered to the casein micelles and the reduction in frictional resistance and surface area (Jeurnink and Kruif, 1993; Kristensen et al., 1997). The differences in viscosity among different breeds presented a good consistency with the milk composition (Table 1).

Viscosity of milk samples (measured using a Ostwald capillary viscometer) as a function of temperature. Symbols: •, Murrah; Δ, F1; ▴, FH. Results are the average of three repeated measurements with the error bars indicating the standard deviation.
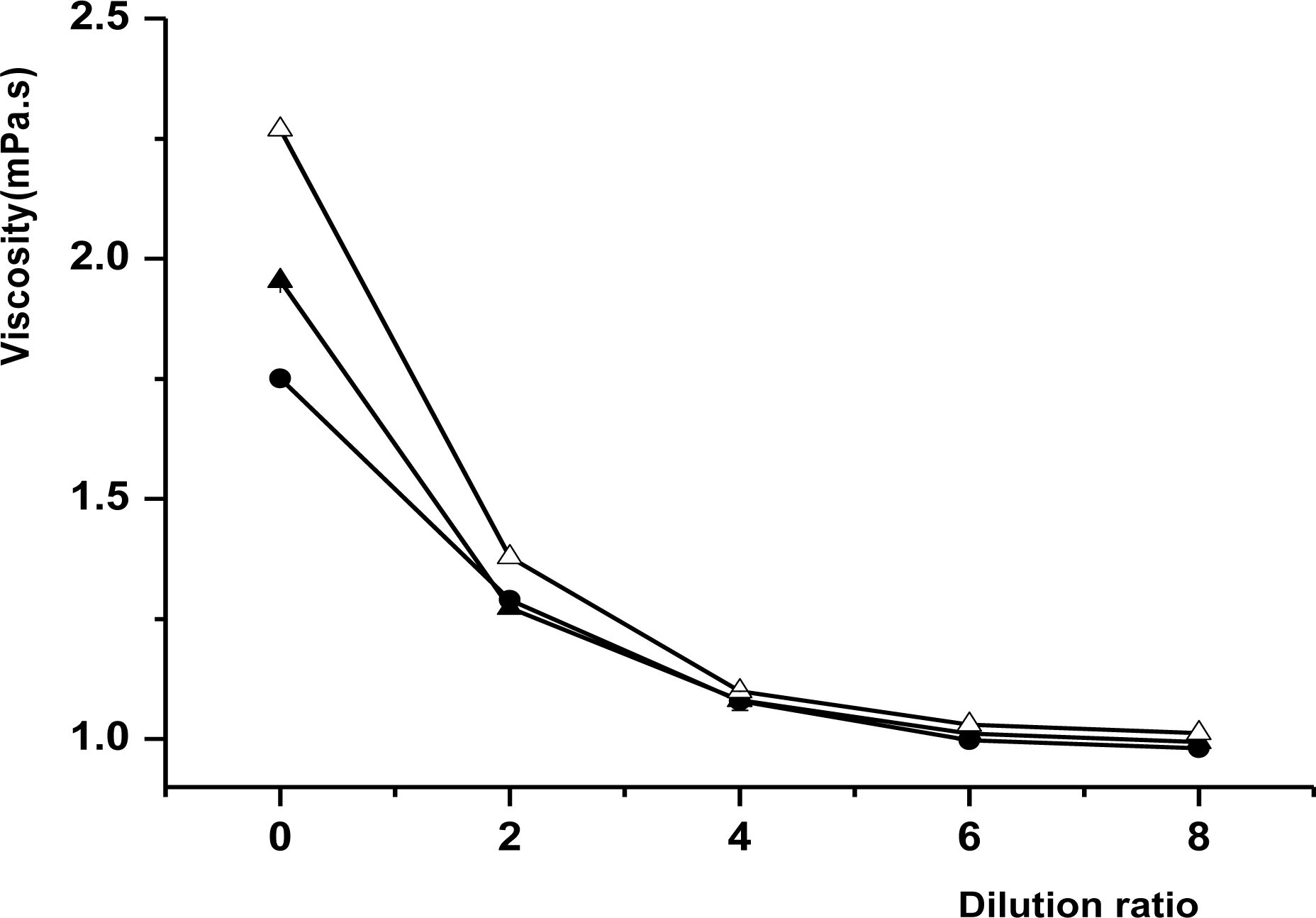
Viscosity of milk samples (measured using a Ostwald capillary viscometer) as a function of dilution ratio. Symbols: •, Murrah; Δ, F1; ▴, FH. Results are the average of three repeated measurements with the error bars indicating the standard deviation.
The viscosity of all milks showed the same trend of reduction with the decreasing milk concentration as follows: F1>FH>Murrah (Figure 6). There were significant differences (p<0.05) in viscosity among the three breeds at high concentrations (25% to 100%); the viscosity of F1 was significantly (p<0.05) higher than that of FH, whereas only slight differences were observed in low concentrations (12.5% to 25%).
Effect of pH and concentration of three cattle breeds on milk zeta potential
Zeta potential has been widely used to assay the magnitude of charge content on colloidal particles and evaluate the stability of colloidal dispersion (Anema and Klostermeyer, 1996; Morrison and Ross, 2002; Duan et al., 2011). Zeta potentials of milk samples were significantly (p<0.05) affected by pH value (Figure 7). The correlation coefficients between Zeta potentials and pH value analyzed by correlation analysis were −0.995, −0.972, and −0.986 for Murrah, FH, and F1, respectively. It indicated that milk zeta potentials were evidently affected by varying pH, as clearly represented by river buffalo milk.
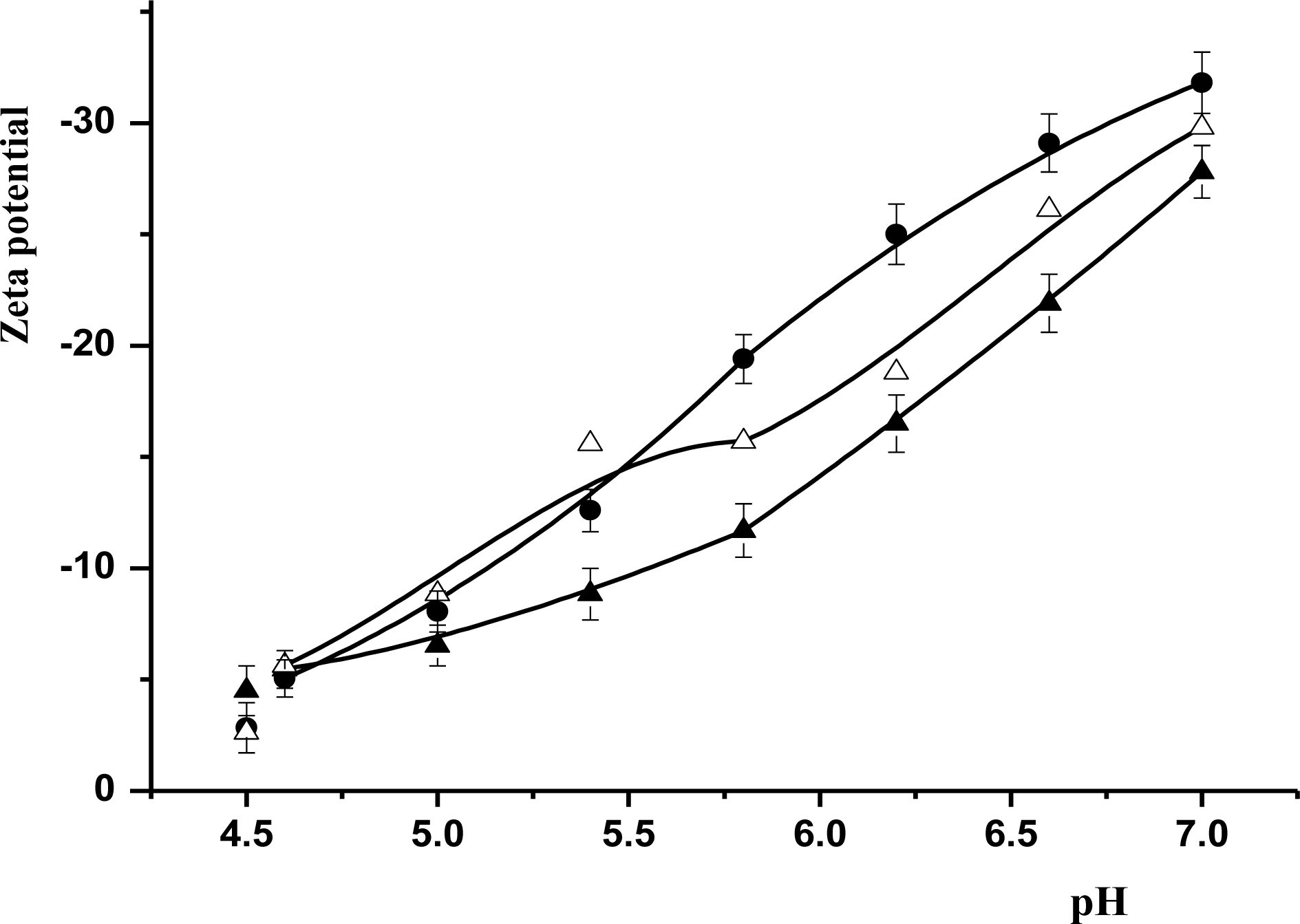
Zeta potential of milk samples as a function of pH. Whole samples were conducted by laser Doppler electrophoresis with the Nano-S Zetasizer (Malvern Instruments Limited, Malvern, Worcestershire, UK) after diluted 200 times with Milli Q-water. Symbols: •, Murrah; Δ, F1; ▴, FH. The data presented are averages of three replications with the error bars indicating the standard deviation.
Figure 7 shows that the absolute value of zeta potential of the three local kinds of buffalo milk decreased linearly with the reducing pH (from 7.0 to 4.5). Figure 7 shows that the absolute zeta potential of milk suspension of Murrah was remarkably (p<0.05) higher than that of FH. It is noteworthy that the average absolute zeta potential of Murrah milk was 6.85 mV above that of FH and 3.73 mV higher than that of F1 at pH 5.8 to 7.0. However, the absolute zeta potential of F1 milk suspension was slightly higher than that of Murrah at pH 4.6 to 5.6.
Zeta potentials of all milk samples as a function of dilution ratio are shown in Figure 8. The absolute value of zeta potential of the three local kinds of buffalo milk decreased with the reducing concentration in the initial stage, then increased, but finally decreased. The absolute value of zeta potential of all the samples reached the maximum while the milk were diluted 6 times. Meanwhile, it also can be seen that the absolute value of zeta potentials of the three local kinds of buffalo milk were different, and F1 was higher than FH, followed by Murrah (Figure 8). It indicated that the three local kinds of buffalo milk dispersion system was much more stable when they were diluted at 6 times, and F1 was the most stable dispersion system. This may relate to the different dry matter content and the casein micelles distribution.
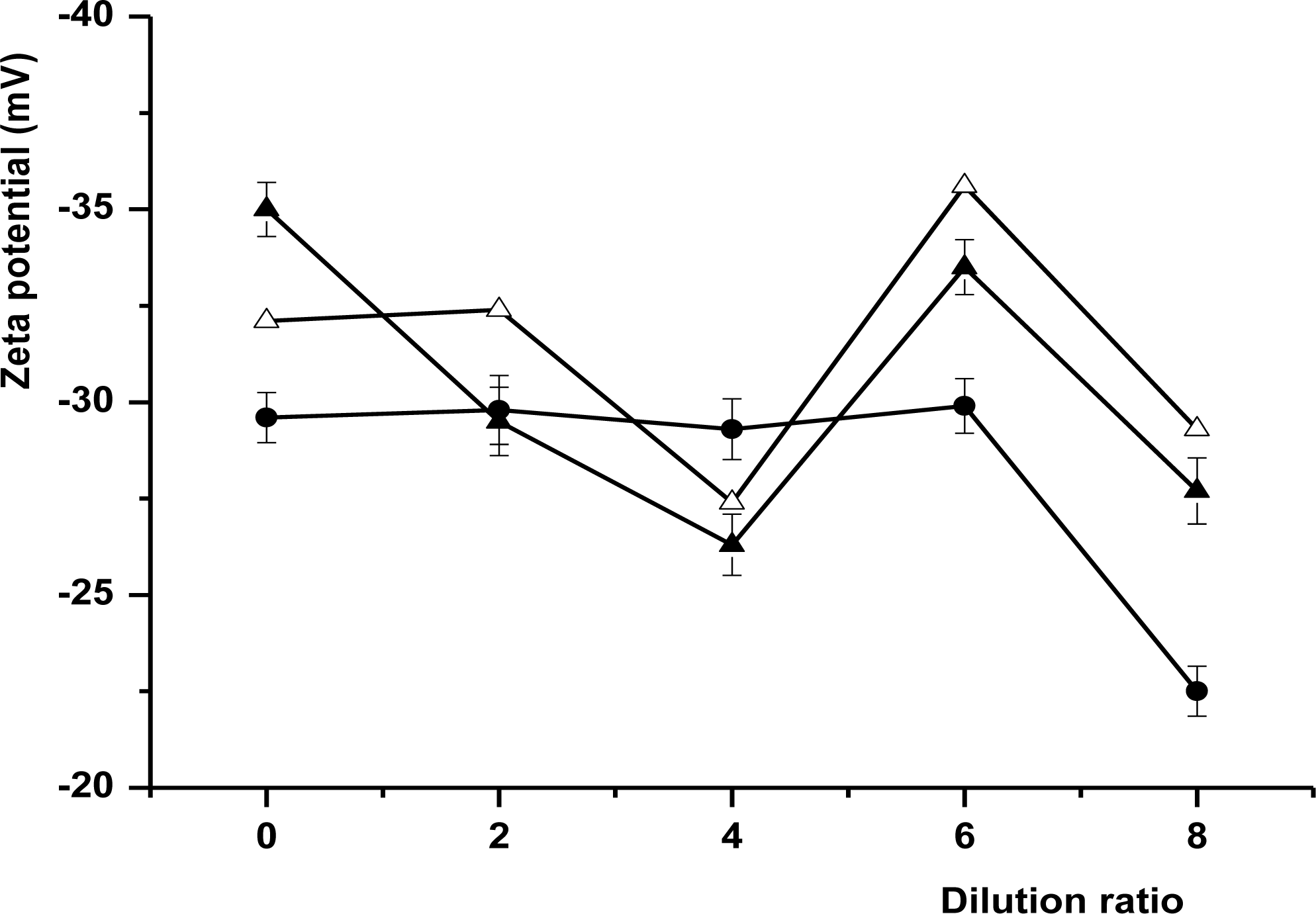
Zeta Potential of milk samples as a function of dilution ratio. Whole samples were conducted by laser Doppler electrophoresis with the Nano-S Zetasizer (Malvern Instruments Limited, Malvern, Worcestershire, UK) after diluted 200 times with Milli Q-water. Symbols: •, Murrah; Δ, F1; ▴, FH. Results are the average of three repeated measurements with the error bars indicating the standard deviation.
With the reducing pH, surface charge of colloidal particles increased, which enhanced the intermolecular electrostatic repulsion among proteins and resulted in more stable state. Some works have been done in this area. Bouzid et al. (2008) found that the zeta potential of skim bovine milk varies linearly with the pH, from −4 to −24 mV between pH 5.0 and 6.7, respectively. After that, observations on zeta potential of the Mediterranean buffalo milk fat globules and Switzerland cow milk fat globules under the native pH were also reported (Ménard et al., 2010). This work has demonstrated that the dispersion stability of river buffalo milk was superior to that of hybrid offspring milk, which may be related to the higher Ts content in F1 milk. Increasing particle size contributes to the aggregation of casein with denatured whey protein and the interaction between casein micelles and whey protein under reduced pH (Klein et al., 2010; Kehoe and Foegeding, 2011). Other milk compositions could be contributors too, such as bovine lactoferrin, fat globule membrane, etc. Bengoechea et al. (Bengoechea et al., 2011) used zeta potential changes to reveal the changes of conformation, aggregation, and electrical charge of bovine lactoferrin. Michalski suggested that a significant increase in zeta potential indicated the incorporation of caseins into the milk fat globule membrane (Michalski et al., 2006).
Zeta potential in the present study did not reach zero at the isoelectric point as described (Bouzid et al., 2008), but it was in accordance with the results of Anema and Klostermeyer (1996). These results can be attributed to the influence of ionic strength on the electrostatic interactions in the system, and the electrostatic repulsion between individual proteins was reportedly reduced (Bengoechea et al., 2011).
Casein of milks from three cattle varieties
Proteins of three types of milk were detected by SDS-PAGE (Figure 9). Buffalo αs-casein had a slightly faster mobility than standard αs-casein and seemed to be lack of one kind of αs-casein, which is worth of further investigations. Buffalo β-casein had a slightly slower mobility than standard β-casein. There is no significant difference between buffalo caseins. It indicated that milk protein and molecular weight was greatly related to cattle breeds. Nevertheless, the differences between the caseins of hybrid offspring and their male parent could be ignored. αs1-casein is the major allergen (Elsayed et al., 2004), further study on which is helpful to the development of hypoallergenic or non-allergic dairy products from Chinese buffalo milk.
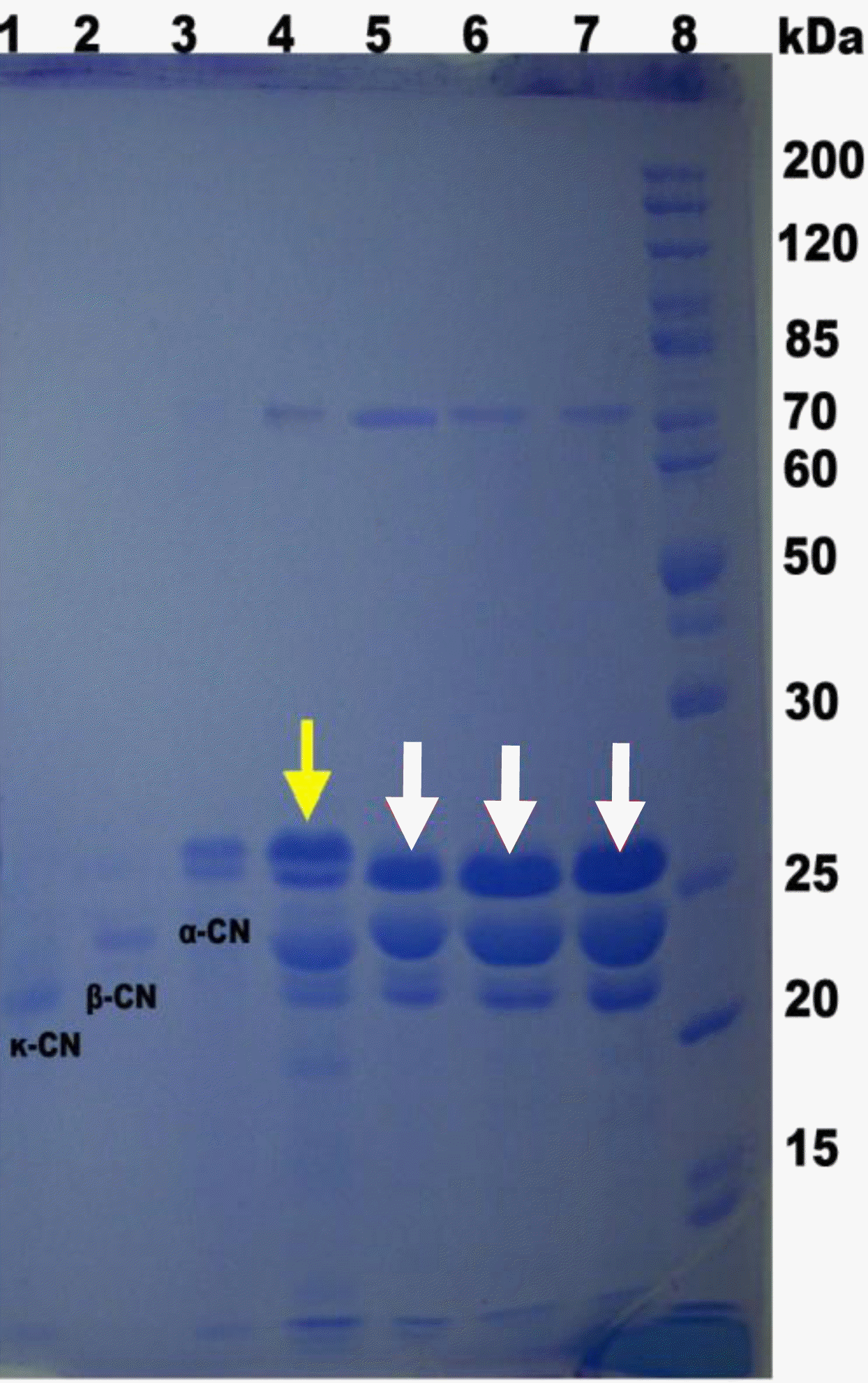
SDS-PAGE of milk protein samples. Electrophoresis was carried out with a vertical electrophoresis system and on 12% acrylamide separating gel and 4% stacking gel with the help of standard mixtures of marker proteins. Lane 1: standard of κ-casein (κ-CN), Lane 2: standard of β-casein (β-CN), Lane 3: standard of α-casein (α-CN), Lane 4: Holstein milk as a reference, Lane 5: Murrah milk, Lane 6: FH milk, Lane 7: F1 milk, Lane 8: molecular weight markers 10 to 200 kDa (Fermentas-#SM0661).
CONCLUSION
By comparing the three cattle breeds for bovine milk composition and physicochemical properties, this work demonstrated that the breeds had evident effect on milk composition and physicochemical properties. Overall, the nutritional value of river buffalo milk was lower than that of swamp buffalo and their hybrid offspring. However, the milk of crossbreed multi-generation was more like that of their paternal strain, and its nutritional value was lower than that of F1, so were the physicochemical properties. There were no clear differences in milk protein molecular weight between Murrah, F1, and FH. Comparing with river buffalo milk, the technological characteristics of hybrid offspring buffalo (FH) milk are conducive for further utilization of their nutritional value. This work provides a reference to improve buffalo varieties for milk production and to produce different types of dairy products from different types of milk, which warrants further studies.
Acknowledgements
The work was supported by the National Natural Science Foundation of China (Grant No. 31071576) and Talents Project of Guangxi University (Grant No. XZL090325), P. R. China.


