Immune Response of Broiler Chickens Fed Diets Supplemented with Different Level of Chromium Methionine under Heat Stress Conditions
Article information
Abstract
The objectives of this study were to investigate the immune responses of broiler chickens fed diets supplemented with different level of chromium methionine (CrMet) in heat stress (HS) condition. Two hundred and eighty eight male broiler chickens (Ross 308) were allocated to four treatment groups (supplementation with 0, 200, 400 or 800 ppb Cr in the form of CrMet) in a completely randomized design. The experiment was conducted at heat stressed condition and all birds were kept under temperature of 33±2°C. Antibody titers against Newcastle disease virus (NDV) and infectious bronchitis virus (IBV), heterophil to lymphocyte ratios (H/L), and concentration of plasma cortisol (CPC) were measured at 21 and 42 d. At 42 days of age two birds were chosen randomly from each replicate, slaughtered, spleen and bursa of Fabricius were collected, weighed and expressed as a percentage of live body weight. Antibody titers against NDV and IBV at 21 and 42 days of age in broiler fed supplemental CrMet were higher than in broiler chickens fed control diet (p<0.05). CPC level in broiler chickens fed CrMet were significantly (p<0.05) decreased. Increases in lymphocyte counts and consequently a decrease in heterophil to lymphocyte ratios in broiler chickens fed 800 ppb Cr were observed at 21 and 42 d. Supplementation with CrMet had no significant effect on lymphoid organs of broilers. The results suggest that dietary CrMet supplementation at a level of 800 ppb can improve some immune responses of broiler chickens under heat stress conditions.
INTRODUCTION
Heat stress (HS) is of great concern in the poultry industry. Feed efficiency, growth rate, mortality, and other important traits governing productivity in the poultry industry are adversely affected by severe HS. It has been established that high environmental temperatures affect the development of a specific immune response in chickens (Thaxton et al., 1968; Thaxton and Siegel, 1972). Chromium is a component of glucose tolerance factor (GTF) and is important in carbohydrate, fat, and protein metabolism presumably by potentiating the action of insulin (Anderson, 1987; Mertz, 1993). Stress and disease increased urinary excretion of Cr (Pekarek et al., 1975; Borel et al., 1984; Anderson et al., 1988) and may exacerbate a marginal Cr deficiency. Chromium supplementation has shown be effective in diminishing adverse effects of stress, reducing cortisol levels and improving immunity (Chang and Mowat, 1992; Kegley and Spears, 1995). Frequently these factors become important in enhanced growth performance (Moonsie-Shageer and Mowat, 1993). Improvements in immune response have been observed when organic forms of Cr were supplemented to broilers (Luo et al., 1999), stressed feeder calves (Chang and Mowat, 1992; Moonsie-Shageer and Mowat, 1993) and dairy cows (Burton et al., 1993). No recommendations for Cr are currently listed for poultry (NRC, 1994, 1997) and most poultry diets are basically composed of plant origin ingredients, which have usually a low content of Cr (Giri et al., 1990). Chromium-L-methionine is a newly available organic chromium source whose bioavailability and effects have not been previously determined in broiler chickens. This study was conducted to investigate the effects of different levels of Cr methionine on immune responses in heat-stressed broiler chickens.
MATERIALS AND METHODS
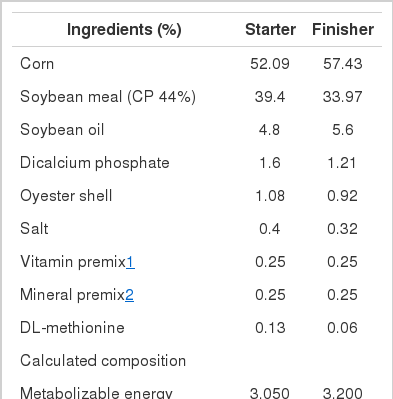
Two hundreds and eighty eight one-day-old male broiler chickens (Ross 308) were allocated to four treatments in a completely randomized design. Dietary treatments supplemented with 0 (control), 200, 400 and 800 ppb Cr in the form of Cr- methionine (contain 1,000 mg Cr kg−1). Each dietary treatment was randomly allocated to six replicates of 12 chicks each. Chicks were raised from 1 to 42 days of age, in controlled house with mean value of daily temperature 33±2°C. The chickens were fed a maize-soybean meal starter diet (219 g protein, 3,050 kcal ME kg−1) up to 21 days and a finisher diet (200 g protein, 3,200 kcal ME kg−1) up to 42 days. The basal diets in mash form were formulated to meet or exceed the nutrient requirements of broiler chickens (NRC, 1994). Cr contents were 3.39 and 3.85 ppm in starting and finishing basal diets, respectively, as measured by atomic absorption spectrometer with a graphite furnace (Perkin-Elmer, AAnalyst 600, USA). The diets and fresh water were provided ad libitum. Ingredients and chemical composition of the starter and finisher basal diets are shown in Table 1.
Live infectious bronchitis disease vaccine (H120) was administered orally (drinking water) at 4 and 13 d. All chicks were intramuscularly immunized with killed vaccine of Newcastle viruses at age of 9 days.
On days 21 and 42 blood samples were collected from the wing vein of two birds per replicate (using EDTA as anticoagulant). Samples were centrifuged at 3,500 g for 15 min to determine plasma antibody titers against Newcastle and infectious bronchitis disease viruses. Antibody titers against Newcastle disease virus were determined by haemagglutination inhibition (HI) test and were expressed as the logarithm base 2. Antibody titers against infectious bronchitis disease virus were determined by enzyme linked immunosorbent assay (ELISA) (IDEXX Inc., Westbrook, ME 04092, USA).
Blood smears were prepared using May-Greenwald-Giemsa stain and heterophil to lymphocyte ratios were based on a total of 100 cells (Gross and Siegel, 1983).
Plasma cortisol concentration measured by cortisol Elisa kit (RE52061 Westbrook, ME, USA). The result was monitored as OD at 450 nm and cortisol concentrations (mg/ml serum) were calculated from a standard curve as suggested by the company. Two birds from each replicate were slaughtered on day 42 and lymphoid organs such as spleen and Bursa of Fabricius were collected, weighed and expressed as a percentage of live body weight.
Data were analyzed by analysis of variance procedures appropriate for a completely randomized design using the GLM procedures of SAS (2002). Significant differences (p<0.05) among treatment means were determined using Duncan’s new multiple range test.
RESULTS AND DISCUSSION
The effects of supplemental Cr on plasma cortisol levels, antibody titers against Newcastle and infectious bronchitis virus are shown in Table 2. Antibody titers against NDV and IBV at 21 and 42 days of age in broiler fed supplemental Cr were higher than in broiler chickens fed control diets (p<0.05). Elevated antibody titers against NDV were reported in broiler chickens under heat stress with supplement of 2 or 10 ppm Cr, either in the form of CrCl3 or yeast (Guo et al., 1999). Lee et al. (2003) reported that antibody titers against NDV was improved in broiler chickens fed 400 ppb Cr picolinate. Improved immune responses against virulent antigen were also reported in weanling pigs (Lee et al., 1997) with a dietary Cr supplement. CPC in broiler chickens fed Cr supplementation was lower than chickens fed a control diet. These results are in agreement with most experiments involving supplemental Cr. A reduction in CPC of heat-stressed broilers fed Cr picolinate was reported by Sahin et al. (2002). Chang and Mowat (1992) observed CPC decreased in stressed steers supplemented with Cr from high-Cr yeast. However, there are also a number of reports of no effect of Cr supplementation on serum cortisol levels (Kegley and Spears, 1995; Pollard et al., 2002).

Effect of chromium supplementation on antibody titer against Newcastle and infectious bronchitis virus and cortisol levels at different ages of broiler
Various stressors, including cold exposure (Sasaki and Weekes, 1986), short exposure to heat (Christison and Johnson, 1972), isolation (Hashizume et al., 1994), and transportation (Arave et al., 1988) increase plasma cortisol. High concentrations of Cr from CrPic can directly inhibit cortisol secretion in an agonist-stimulated adrenocortical cell line (Kim et al., 2006). Zulkifli et al. (2000) reported antibody production in young broiler chickens was decreased in HS condition. This reduction could be indirectly due to an increase in inflammatory cytokines under stress, which stimulates the hypothalamic production of corticotrophin releasing factor (Ogle et al., 1997). Corticotrophin releasing factor is known to increase adrenocorticotropic hormone from the pituitary; adrenocorticotropic hormone then stimulates corticosterone production from the adrenal gland and corticosterone inhibits antibody production (Gross, 1992).
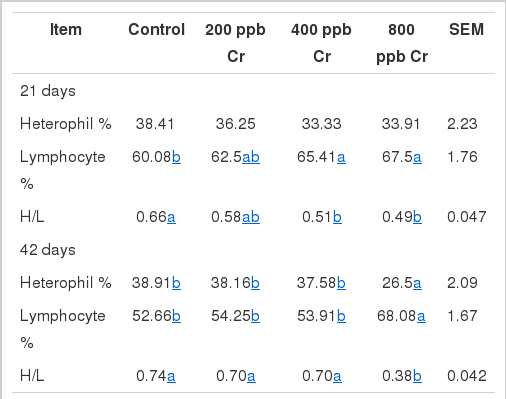
In this study, the increases in lymphocyte count and consequently decrease in heterophil to lymphocyte ratios in broiler chickens fed 800 ppb Cr were observed at 21 and 42 d (Table 3). The heterophil to lymphocyte ratio has been accepted as a reliable index for determining stress in poultry (Gross and Siegel, 1983; McFarlane and Curtis, 1989). It has also been found that exposure of birds to HS results in an increase in the heterophil to lymphocyte ratio (Altan et al., 2003; Zulkifli et al., 2003). Gross and Siegel (1983) found that the number of heterophils increased in the blood of corticosterone fed chickens. The increases in lymphocyte counts and decreases in heterophil to lymphocyte ratios by Cr supplementation in heat stressed chickens in the present study may be attributed to decreased glucocorticoid secretion.
The exact mechanism by which Cr enhances the immune system is not known. However, one of the consistent results of the studies was that Cr reduced plasma cortisol levels. Cortisol, the most important glucocorticoid, has been found to be immunosuppressive, inhibiting the production and actions of antibodies, lymphocyte function, and leucocyte population (Roth and Kaeberle, 1982; Munck et al., 1984).
Supplementation with CrMet had no significant effect on lymphoid organs of broilers (Table 4). However, all organ weights were significantly reduced when birds were exposed to HS (p<0.05). Toghyani et al. (2007) observed that supplementation with different levels of Cr-picolinate in the diet of heat-stressed broilers had no effect on lymphoid organs. Bartlett and Smith (2003) suggest that the decrease in lymphoid organ weights could have been a result of the reduction in feed intake, thereby providing fewer nutrients for proper development of these organs under HS conditions.

Effect of chromium supplementation on lymphoid organ weight (percentage of live body weight) at 42 days of age
Overall, it appears that dietary CrMet supplementation at level of 800 ppb can improve some immune responses of broiler under HS condition. More extensive research is needed to determine the effect of Cr-Methionine on growth performance and immune response in broilers under varying rearing conditions.