Combination of an Enzymatically Hydrolyzed Yeast and Yeast Culture with a Direct-fed Microbial in the Feeds of Broiler Chickens
Article information
Abstract
A balance trial experiment was carried out to evaluate the potential relationship between an enzymatically hydrolyzed yeast (EHY) and yeast culture combined with a live Bacillus subtilis (Bs) on the productive parameters, ileal digestibility, retention of nutrient and energy and villus morphology in broilers. Seventy two 28 d old, Ross B308 male broilers were assigned to a factorial combination of 2 levels of EHY (0 and 1 kg/ton of feed) and 2 levels of Bs (0 and 125 g/ton of feed). The experiment lasted 2 weeks. Several treatment interactions were observed. EHY-fed broilers showed the lowest feed intake and feed conversion ratio whereas Bs-fed broilers showed the highest feed intake and intermediate feed conversion ratio (EHY and BS interaction, p<0.05). Also, EHY-fed broilers had greater ileal digestibility of dry matter (EHY and BS interaction, p<0.01) and energy (EHY and BS interaction, p<0.05) but these responses were counterbalanced by the combination of EHY and Bs. The thickness of the mucosa was similar between the control and EHY-fed broilers, but was lowest when Bs was added alone (EHY and BS interaction, p<0.01). The thickness of the villus was greater in EHY plus Bs-fed broilers, intermediate for the control and lower for Bs or EHY-fed broilers (EHY and BS interaction, p<0.05). The area of the villus was greater in the control and EHY plus Bs-fed broilers (EHY and BS interaction, p<0.05). In addition, EHY-fed broilers showed greater breast yield and nitrogen retention (p<0.01) and ashes digestibility (p<0.05). On the other hand, Bs-fed broilers had greater carcass and breast weight, nitrogen retention, energy excretion and villus height (p<0.05). In summary, EHY and Bs enhanced some growth, carcass and nutrient retention responses, but did not show any synergic relationship in these responses. Opposite to this, the results suggest that the positive effect of EHY on the feed conversion and digestibility of nutrients were counterbalanced by the addition of Bs.
INTRODUCTION
For many years it has been recognized that the use of antibiotics growth promoters (AGP) enhance the efficiency of growth and health of poultry. However, potential dangers associated with the development of resistant pathogenic bacteria after long use of AGP have also been documented, forcing poultry industry to consider other biologically safe alternatives (Ratcliff, 2000; Ferket et al., 2002). Currently, there is considerable evidence showing that the inclusion of mannanoligosaccharide (MOS) in poultry feeds improves the growth and feed conversion ratio, and is considered one of the best options for AGP replacement (Hooge, 2004; Rosen, 2007). Some advantages of MOS over AGP are: i) No withdrawal period required before slaughter; ii) No residual effect on poultry products; and iii) No bacterial mutation which could lead to resistance in pathogenic bacteria.
It is proposed that the benefits of MOS is due to the presence of mannose units that binds potential pathogenic bacteria, particularly those with type 1 fimbria on their surface, blocking their adhesion and colonization of the intestinal cells (Mul and Perry, 1994). It has been suggested that MOS favors the growth of beneficial bacteria such as Lactobacillus which are capable of neutralizing some enterotoxins and inhibiting the growth of pathogenic bacteria like E. coli, Clostridium and Streptococcus by producing organic acids and reducing the intestinal pH (Spring et al., 2000). This can lead to better gut health and increased nutrient availability resulting in enhanced growth and health of poultry (Radecki and Yokoyama, 1991; Stanley et al., 2004). There is also considerable evidence showing that whole cells and several derivatives from yeast such as yeast culture (YC), yeast extracts (YE) and yeast fermentation products, that retain the cell wall components, exert similar beneficial effects as MOS on the growth and feed conversion ratio of poultry (Stanley et al., 2004; Zhang et al., 2005; Gao et al., 2009).
On the other hand, some authors have reported that the combination of an oligosaccharide such as MOS and an appropriate probiotic (symbiotic products) could increase the efficacy above that of both products used separately (Bailey et al., 1991; Fukata et al., 1999; Sun et al., 2004). Bacillus is one of the microorganisms used in broilers as direct-fed microbial (DFM), which also favor the development of Lactobacillus and depresses the growth of pathogenic bacteria such as E. coli, Clostridium, Streptococcus and Salmonella leading to improved growth and feed conversion ratio of poultry. This information suggests that both, MOS and Bacillus, enhance the proliferation of beneficial microbes, and inhibit the multiplication of harmful microbes in the gut.
It is probably that MOS and Bacillus together may increase the digestibility of feeds since the first may increase the disaccharidase activity on the intestinal brush-like border, and the second, may release digestive enzymes during the spore germination (Gedek, 1999). By stimulating the proliferation of Lactobacillus, MOS and Bacillus may also help the digestion process since some research has shown that Lactobacillus increases the level of amylase in the intestine of host chickens, helping the starch digestion (Jin et al., 2000) and is, itself, a good source of digestive enzymes such as amylase, protease and lipase (Panda et al., 2000).
It has been reported that MOS and Bacillus may increase the intestinal absorptive area by increasing the villus height in different portions of the gut (Macari and Maiorka, 2000; Loddi, 2003; Pelicano et al., 2003) resulting in higher nutrient digestion and absorption. It has been reported as well that MOS and Bacillus combined may reduce the depth of the crypt, due to a lower enterocyte replacement rate, and by increasing the villus density in the duodenum (Pelicano et al., 2005b). This suggests that combining MOS and Bacillus may lead to an improvement in health, growth, and digestive processes.
Since there are several sources of MOS, yeast derivatives, and Bacillus (or DFMs) commercially available for broilers, it is important to test the effectiveness of these products against each other or in combination. Therefore, the main issue addressed in the present study was to elucidate if there was any additive or synergistic relationship between a product containing yeast derivatives (Celmanax®) and a product containing a live Bacillus subtilis (GalliPro®) in different digestive responses.
Celmanax® is a trade mark of Vi-Cor® (Mason City, IA, USA) that contains enzymatically hydrolyzed yeast (EHY), YE and YC. Celmanax® also contains yeast mannans and provides the metabolites normally found in YC and can replace yeast-based products in the diet. Heat-tolerant GalliPro® is a registered trademark owned by Chr. Hansen (Hoersholm, Denmark) which is a probiotic feed ingredient, also called DFM. GalliPro® is based on the naturally-occurring superior high activity strain of Bacillus subtilis. Therefore, the present balance experiment was carried out to evaluate the potential relationship between an enzymatically hydrolyzed yeast (EHY) and yeast culture combined with a live Bacillus subtilis (Bs) on the production, ileal digestibility, retention of nutrient and energy, and villus morphology in broilers.
MATERIALS AND METHODS
Birds and treatments
In the experiment, 72 (28 days of age) Ross B308 male broilers individually housed in holding mesh-floored cages with a metal feeder and a cup drinker in a naturally ventilated unit were used. Broilers were randomly allotted to four treatments in a complete randomized design with a factorial combination of 2 levels of EHY (0 and 1 kg/ton of feed) and 2 levels of Bs (0 and 125 g/ton of feed). Monensin (500 g/ton) was added to all diets. Diets were formulated according to the nutrient recommendations of Ross for growing broilers (Table 1).
Management and sampling
Water was provided ad libitum. Broilers were weighed at the beginning and end of the 2 wk trial. Feed was offered ad libitum during the first 10 days. Then feed allowance was limited to 90% of the ad libitum intake during the last four days of the trial and in this period, excreta were totally collected. Feed offered during the excreta collection was added with 0.25% chromic oxide as internal marker for determination of the ileal digestibility of nutrients. On the last day of the trial, all broilers were killed by cervical dislocation. The ileum was excised and its content was collected in polyethylene bags and immediately stored on ice. The ileum was considered the portion of the small intestine limited by the Meckel’s diverticulum and the ileocecal valve. The ileal contents from three broilers per treatment were pooled and each one of these samples was considered one observation. A portion of 5 cm of duodenum was obtained from eight broilers per treatment. The duodenum content was rinsed with 10% formalin and the end points were tied up with silk. Then, 2 ml of formalin were injected to each portion and placed in plastic bottles filled with formalin. Carcass was dissected and its main components (breast, legs and thighs) were rinsed with clean water, dried and weighed.
Laboratory determinations
Excreta and ileal contents were lyophilized and ground using a 2 mm mesh. Determinations of dry matter, ashes, nitrogen, and energy were done in excreta, ileal contents and diets following standard procedures (AOAC, 1992). Chromium was also analyzed in diets and ileal contents. Then, the ileal digestibility and retention of nutrients were estimated. Duodenal portions were fixed in formalin, dehydrated with an alcohol-xylene sequence, and embedded in paraffin. Three pieces of 5-μm slices were prepared and stained with hematoxylin-eosin. Mucosa thickness, villus height and thickness and crypt depth from each slice were measured (μm). The villus area and villi height/crypt depth ratio (VCR) were also estimated. Readings were carried out under light microscope. About 15 measurements were done per bird.
Statistical analysis
Results were subjected to ANOVA by using the GLM procedures (SAS, 1995). For growth performance, carcass measurements, nutrient retention and villi morphology the experimental unit was each broiler, but for the ileal digestibility of nutrients, the experimental unit was a group of three broilers. Percentage results were transformed to arcsine values before analysis.
RESULTS
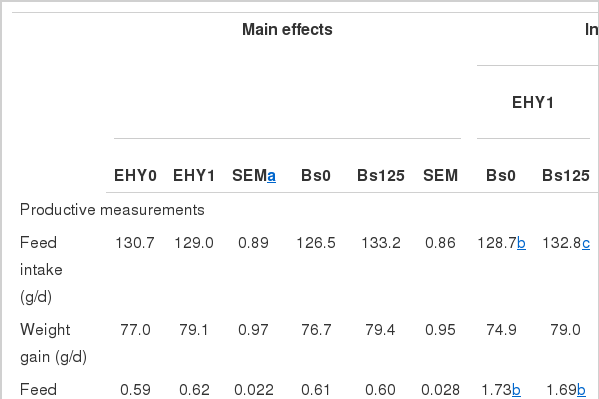
Growth performance and carcass measurements
The EHY and Bs interaction was significant for feed intake and feed conversion ratio (Table 2). When EHY was added alone, broilers showed the lowest feed intake and feed conversion ratio whereas Bs-fed broilers showed the highest feed intake and intermediate feed conversion ratio (EHY and BS interaction, p<0.05). EHY-fed broilers, either alone or combined with Bs, also showed a greater breast yield (p<0.01).
Productive and carcass measurements in broilers fed an enzymatically hydrolyzed yeast and yeast culture combined with a live Bacillus subtilis
Bs-fed broilers showed a trend for greater weight gain (p<0.10). The carcass and breast weight were improved (p<0.05) and there was a non-significant trend for greater carcass yield (p<0.10) with Bs in comparison to the control group.
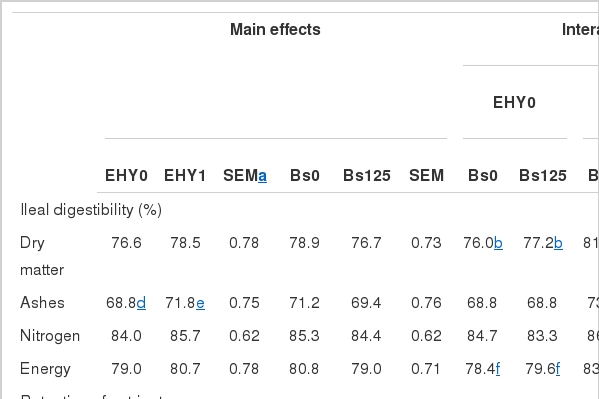
Ileal digestibility
The EHY and Bs interaction was significant for the ileal digestibility of dry matter and energy (Table 3). EHY-fed broilers had greater ileal digestibility of dry matter (EHY and BS interaction, p<0.01) and energy (EHY and BS interaction, p<0.05) but these responses were counterbalanced by the combination of EHY and Bs. EHY-fed broilers showed higher (p<0.05) ashes digestibility. There was also a trend (p<0.07) for higher ileal nitrogen digestibility in EHY-fed broilers compared to the control group.
Nutrient retention
EHY-fed broilers had improved nitrogen retention (p<0.01) and a trend for lower energy excretion (p<0.09; Table 3). Bs-fed broilers showed a positive trend of lower (p<0.07) dry matter and nitrogen excretion and had greater (p<0.05) nitrogen retention and lower (p<0.05) energy excretion compared to the control group.
Villi morphology
The EHY and Bs interaction was significant for several measurements (Table 4). The thickness of the mucosa was similar between the control and EHY-fed broilers, but was lowest when Bs was added alone (EHY and BS interaction, p<0.01). The villus height was similar between the control and EHY-fed broilers, but there was a trend of being lower when Bs was added alone (EHY and BS interaction, p<0.07). The thickness of the villus was greater in EHY plus Bs-fed broilers, intermediate for the control and lower for Bs or EHY-fed broilers (EHY and BS interaction, p<0.05). The area of the villus was greater in the control and EHY plus Bs-fed broilers (EHY and BS interaction, p<0.05).

Histological measurements of the duodenum in broilers fed an enzymatically hydrolyzed yeast and yeast culture combined with a live Bacillus subtilis
EHY-fed broilers showed non-significant trends of higher thickness of the mucosa (p<0.10) and depth of the crypts (p<0.08). Whereas Bs-fed broilers had greater thickness of the mucosa (p<0.01), height of the villi and depth of the crypts (p<0.05).
DISCUSSION
An improved feed conversion ratio shown by EHY-fed broilers is in agreement with previous findings observed in broiler fed diets supplemented with yeast. In a meta-analysis of trials of MOS-fed broilers a positive effect of MOS was observed in 80% of the studies, with an average relative improvement of 1.6 and 1.99% in weight gain and feed conversion ratio, respectively (Hooge, 2004). In this experiment, the feed conversion ratio was improved by 4.4% in EHY-fed broilers. In a recent publication from our laboratory, a 6.9 and 3.8% improvement in weight gain and feed conversion ratio was reported in broilers fed the same EHY product, from 35 to 49 d of age, as that used in the present research (Gomez and Angeles, 2011). Improved growth performance have been reported in broilers fed YC, YC residue, whole cells, cell wall components or a fermentation product from yeast (Stanley et al., 2004; Zhang et al., 2005; Gao et al., 2008, 2009). The greater breast yield in EHY-fed broilers is in agreement to other results observed in broiler fed yeast added diets. Greater weight and yield of the carcass and breast was reported by Gomez and Angeles (2011) in EHY-fed broilers. Clementino dos Santos (2002) reported increased breast yield in MOS-fed broilers. The addition of dried yeast in broilers resulted in higher carcass weight and yield (Adejuno et al., 1999; Onifade et al., 1999; Miazzo et al., 2005). Also, the inclusion of live yeast increased the carcass yield on free-range chicken (Pelicia et al., 2004).
In several studies, substantial improvements in weight gain and feed conversion of broilers supplemented with different types of Bacillus have been observed (Santoso et al., 1995; Gil-Turnes et al., 2007; Opalinzki et al., 2007). In a research including four floor experiments, improvements were also reported in body weight gain and feed conversion ratio in Bs-fed broilers (Hooge et al., 2004). These findings agree with our results since Bs-fed broilers showed better performance and carcass yield compared to the control group. On the other hand, Pelicano et al. (2004; 2005a,b) did not find benefits on the growth performance or carcass yield in broilers fed diets added with different Bacillus.
Based on previous research, an increased digestibility of feeds was expected to be obtained by combining MOS and Bacillus. However, this hypothesis did not hold since only EHY-fed broilers showed an increase, whereas Bs-fed broilers showed lower ileal digestibility of nutrients and energy. In previous research, increased disaccharidase activity on the intestinal brush border, as well as tryptophan absorption through the membranes of the jejunum brush border was reported in MOS-fed broilers (Gedek, 1999; Iji et al., 2001). It has also been suggested that MOS has the capability to decrease intestinal colonization of pathogenic bacteria (improving intestinal health and mucosa integrity) and to reduce competition for nutrients between the host and its microflora allowing more nutrients to be available to the host (Iji and Tivey, 1998; Ferket et al., 2002). Probably, a combination of these factors caused the higher ileal nutrient digestibilities in EHY-fed broilers in the present study. Opposite to these results, in other reports no benefits on starch, protein and fat digestibilities were found in MOS supplemented broilers (Kumprecht and Zobac, 1997; Alves et al., 2003; Yang et al., 2008).
The lack of differences in the ileal digestibility of nutrients between the control group and the Bs-fed broilers was unexpected. One of the suggested modes of actions of Bacillus is the release of some digestive enzymes which help to hydrolyze and assimilate the dietary nutrients (Schallmey et al., 2004; Cepero, 2005; Yamabhai et al., 2008). Breves et al. (2000) reported stimulation of glucose uptake in cells from the jejunum of pigs fed with Bacillus cereus. However, in other studies, it has been reported that dietary Bacillus had no effect on the apparent dry matter, nitrogen and energy digestibility in pigs (Spriet et al., 1987; Kim et al., 1993; Scheuermann, 1993). These results were confirmed by Kornegay and Risley (1996) who evaluated two Bacillus-based products (Bacillus subtilis/Bacillus licheniformis, and Bacillus subtilis/Bacillus licheniformis/ Bacillus pumilus). The lack of effects of Bacillus on the nutrient digestibility in pigs agrees with the findings of the present research in broilers.
The greater nitrogen retention and slightly lower energy excretion in EHY-fed broilers matches other results in broilers and turkeys. In a previous experiment, greater nitrogen retention and AMEn was observed in EHY-fed broilers (Gomez and Angeles, 2011). Broilers fed diets supplemented with dried yeast showed higher apparent retention of dry matter, crude protein, ether extract, crude fibre, hemicellulose, cellulose, and neutral detergent fibre than broilers fed a control diet (Onifade and Babatunde, 1996). In MOS-fed broiler, a trend for improved AME (62 kcal/kg) and NE (38 kcal/kg) was observed compared to broilers fed a control diet (Yang et al., 2008). In turkey poults fed YC, improved gross energy and mineral retention was reported (Bradley and Savage, 1995). In male turkeys, the inclusion of MOS increased the AME by 2.94% (Ferket et al., 2002). However, in other studies no differences were reported on the use of energy and other nutrients in MOS-fed broilers (Hughes, 2003; Yang et al., 2007).
The greater nitrogen retention in Bs-fed broilers and the reduction in the excretion of dry matter and energy agree with a report in broilers in which higher dry matter, crude protein and energy retention were observed in broilers fed Bs (Sen et al., 2011). Similar results were reported in pigs (Scheuermann, 1993), however, Kornegay and Risley (1996) did not find any effect in the nitrogen retention of pigs fed two Bacillus-based products.
Positive responses in the villus height in different portions of the gut (Macari and Maiorka, 2000; Loddi, 2003; Pelicano et al., 2003) have been reported in MOS- or Bacillus-fed broilers which may result in greater nutrient digestion and absorption. Supported in these findings, greater thickness of the mucosa and villus height was presumed by combining EHY and Bs. Opposite to this, in the present research, the thickness of the mucosa and villus height were lowest when Bs was added alone and there was not any difference for the rest of the treatments, including the combination of EHY and Bs. The lack of differences in the thickness of the mucosa and the villus height between the control and EHY-fed broilers fits with a previous report by Ferket et al. (2002) who reported that the addition of MOS did not affect the intestinal mucosa or muscularis tissue mass in turkeys, and reports by others researchers (Santos et al., 2004; Yang et al., 2007) in broilers fed MOS. In disagreement with this, Gao et al. (2008) reported a positive linear effect in the villus height in the duodenum of 42 d old broilers with increasing levels of YC. Whereas Pelicano et al. (2005b) reported lower villus height in the duodenum of 21 d old broilers fed a MOS-based prebiotic.
The thickness and area of the villus were numerically greater when EHY and Bs were supplemented together than when they were added independently; however, since these variables were statistically similar between broilers fed the control and the EHY plus Bs, the physiological meaning of these responses are unknown.
It has been reported that MOS and Bacillus combined may reduce the depth of the crypt, due to a lower enterocyte replacement rate (Pelicano et al., 2005b). Opposite to this, the trend for greater crypt depth in EHY-fed broilers seen in the present research suggests a faster enterocyte turnover rate; however no differences were seen on the VCR. Contrary to this, Gao et al. (2008) found that in broilers fed YC the crypt depth was reduced at a low dose but was increased at higher doses, whereas the VCR showed linear increments as the supplementation of dietary yeast increased. Pelicano et al. (2005b) found a reduction of the crypt depth but no effect on VCR in the duodenum of broilers fed a MOS-based prebiotic. The differences observed in the villi morphology of the duodenum on the studies cited may be attributed to differences on the age of the broilers, length of supplementation, type of yeast and stress conditions.
The reduction of the thickness of the mucosa, villus height and depth of the cryps in Bs-fed broilers in comparison to the rest of the treatments, suggests a reduction on the mucosa proliferation (Table 4). These results are opposite to those reported by Pelicano et al. (2005b) in which the villus height was not affected in the duodenum, but was increased in the jejunum and ileum of Bs-fed broilers from 1 to 21 d of age. Also, the results obtained in the present study are not consistent with other reports in which increased villus height and VCR in the duodenum and ileum were reported in broilers supplemented with Bacillus subtilis (Pelicano et al., 2003, 2005b; Samanya and Yamauchi, 2002; Sen et al., 2011) at 21, 35 and 42 d of age.
It has been suggested that taller villi indicate more mature epithelia and enhanced absorptive function due to increased absorptive area of the villi and increased activity of enzymes secreted from the tips of the villi, resulting in improved digestibility (Hampson, 1986; Cera et al., 1988). The present results indicate that even thought EHY-fed broilers had similar villus height and slightly lower villus area in the duodenum than the control group, the ileal digestibility of dry matter, ashes, nitrogen and energy was higher when EHY was supplemented. In the study of Gao et al. (2008) it was reported that higher Ca and P digestibility was not related to a greater villus height and that in some instances the villus height was even lower compared to the control group in the duodenum, jejunum and ileum of 21 and 42 d old broilers fed YC. These findings agree with results of the present experiment.
Furthermore, several authors have reported that the combination of an oligosaccharide and an appropriate probiotic (symbiotic products) may result in higher efficacy than when both products are fed separately (Bailey et al., 1991; Fukata et al., 1999; Sun et al., 2004). In the current research EHY and Bs enhanced some growth, carcass and nutrient retention responses, but did not show any synergic relationship in these measurements. Opposite to this, it was seen that when EHY was added alone the feed conversion and dry matter, ashes and energy digestibility were enhanced, but when EHY and Bs were added together, the responses were negatively affected. These results suggest that the positive effect of EHY on the feed conversion and digestibility of nutrients was counterbalanced by the addition of Bs. The results also show that the responses on the histological measurements of the duodenum were sometime opposite. However, it is important to point out that the combination of EHY and Bs reduced nitrogen excretion by 12% and increased the nitrogen retention by 48%, compared to the control treatment (Table 3), and even though these differences were not statistically significant, these results suggest a possible additive effect of the products in the nitrogen metabolism. All these issues deserve further clarification.
The results indicate that EHY-fed broilers had improved feed conversion ratio, breast yield, ileal digestibility of dry matter, ashes, nitrogen and energy, and nitrogen retention even though they had similar histological duodenal measurements than the control group. The inclusion of Bs improved the feed intake, weight gain, carcass weight and yield, the retention of nitrogen and caused a thinning of the duodenal mucosa. EHY and Bs enhanced some growth, carcass and nutrient retention responses, but did not show any synergic relationship in these responses.