Effects of Pyridoxine on Growth Performance and Plasma Aminotransferases and Homocysteine of White Pekin Ducks
Article information
Abstract
A dose-response experiment with seven supplemental pyridoxine levels (0, 0.66, 1.32, 1.98, 2.64, 3.30, and 3.96 mg/kg) was conducted to investigate the effects of pyridoxine on growth performance and plasma aminotransferases and homocysteine of White Pekin ducks and to estimate pyridoxine requirement for these birds. A total of 336 one-day-old male White Pekin ducks were divided to 7 experimental treatments and each treatment contained 8 replicate pens with 6 birds per pen. Ducks were reared in raised wire-floor pens from hatch to 28 d of age. At 28 d of age, the weight gain, feed intake, feed/gain, and the aspartate aminotransferase, alanine aminotransferase, and homocysteine in plasma of ducks from each pen were all measured. In our study, the pyridoxine deficiency of ducks was characterized by growth depression, decreasing plasma aspartate aminotransferase activity and increasing plasma homocysteine. The ducks fed vitamin B6-deficient basal diets had the worst weight gain and feed/gain among all birds and this growth depression was alleviated (p<0.05) when pyridoxine was supplemented to basal diets. On the other hand, plasma aspartate aminotransferase and homocysteine may be the sensitive indicators for vitamin B6 status of ducks. The ducks fed basal diets had much lower aspartate aminotransferase activity and higher homocysteine level in plasma compared with other birds fed pyridoxine-supplemented diets (p<0.05). According to quadratic regression, the supplemental pyridoxine requirements of Pekin ducks from hatch to 28 days of age was 2.44 mg/kg for feed/gain and 2.08 mg/kg for plasma aspartate aminotransferase and the corresponding total requirements of this vitamin for these two criteria were 4.37 and 4.01 mg/kg when the pyridoxine concentration of basal diets was included, respectively. All data suggested that pyridoxine deficiency could cause growth retardation in ducks and the deficiency of this vitamin could be indicated by decreasing plasma aspartate aminotransferase activity and increasing plasma homocysteine.
INTRODUCTION
Pyridoxine (vitamin B6) and its hydrochlorate salt is usually supplemented to poultry diets as the source of this vitamin. In the early years, the pyridoxine deficiency in ducks was observed by Hegsted and Rao (1945) and the pyridoxine recommendation of NRC (1994) for White Pekin ducks was 2.5 mg/kg. However, since the publication of NRC (1994), further information on duck vitamin B6 requirements was not reported and it is still little for other avian species. Furthermore, due to improved growth rate and the change in feeding conditions of meat poultry compared with the past decades, it is time to reevaluate the requirement of this vitamin.
Pyridoxine can be converted to pyridoxal-5′-phosphate (PLP) and then takes part in the transamination pathway for amino acids as a coenzyme. In chicks, transaminases are sensitive and specific indicators for vitamin B6 status and vitamin B6 deficiency significantly reduces the activities of aspartate aminotransferase and alanine aminotransferase in blood (Cheney et al., 1965; Yen et al., 1976; Oloyo, 2001), but its indicator status has not been reported in ducks. On the other hand, because plasma homocysteine was thought to be a risk factor for cardiovascular disease, vitamin B6 is widely recognized for its importance in the inactivation of homocysteine by serving as coenzyme in the form of PLP for two degradative enzymes (cystathionine β-synthase and cystathionine γ-lyase) in the transsulfuration pathway for homocysteine removal (Selhub, 1999; Miles and Kraus, 2004). In vitamin B6-deficient rats and pigs, plasma homocysteine was significantly elevated (Smolin et al., 1983; Martinez et al., 2000; Lima et al., 2006; Zhang et al., 2009) which indicated that vitamin B6 deficiency may have potential negative effects on vascular health of animals. Therefore, plasma homocysteine may be another new and useful biomarker for vitamin B6 status of animals. However, the effects of vitamin B6 on homocysteine status of ducks and other avian species have not been confirmed.
Herein, considering that the information on vitamin B6 nutrition of modern duck strains is lacking, the objective of present study was to examine the effects of pyridoxine on growth performance and plasma aminotransferases and homocysteine of White Pekin ducks and to determine the pyridoxine requirements for modern duck strains.
MATERIALS AND METHODS
All procedures in our experiments were approved by the animal care and use committee of the Institute of Animal Sciences of Chinese Academy of Agricultural Sciences. A dose-response experiment with seven supplemental pyridoxine levels (0, 0.66, 1.32, 1.98, 2.64, 3.30, and 3.96 mg/kg) was utilized. The basal diet was formulated to be vitamin B6 deficient (Table 1) and it contained 1.93 mg/kg total pyridoxine by calculation according to the data of feed ingredients of the NRC (1994). To produce experimental diets, the basal diet was produced first and then seven experimental diets were constructed by blending the basal diet with different supplemental levels of crystal pyridoxine·HCl containing 82% free base. The supplemental pyridoxine levels of experimental diets were analyzed by high performance liquid chromatography (2695 Alliance, Waters, Milford, MA, USA) with fluorescence detection after extraction by hot water according to the method recommended by the Ministry of Health of China (2010). The analyzed supplemental pyridoxine concentrations in the seven experimental diets were 0, 0.69, 1.33, 1.96, 2.67, 3.35, and 3.99 mg/kg, respectively, which were similar to the calculated pyridoxine values. A total of 336 one-day-old male White Pekin ducks with average body weight of 57±3 g were divided to 7 experimental treatments and each treatment contained 8 replicate pens with 6 birds per pens. These ducklings were reared in raised wire-floor pens (200×100×40 cm) from hatch to 28 days of age. During this period, the birds were given free access to water and feed. Water was provided by drip-nipple and feed was fed in pellet form. In the birdhouse, lighting was continuous and the temperature was kept at 33°C from 1 to 3 days of age and then it was reduced gradually to room temperature until 21 days of age.
At twenty-eight days of age, the weight gain, feed intake, and feed/gain of all ducks from each pen were determined and 2 birds selected randomly from each pen were bled by heart puncture. Blood samples were collected into heparin sodium-anticoagulant tubes and centrifuged at 3,000 rpm for 10 min to obtain plasma. Plasma was kept at −20°C until analyzed. Plasma homocysteine was determined using reversed-phase high performance liquid chromatography (2695 Alliance, Waters, Milford, MA, USA) with precolumn derivation and fluorescence detection according to the method of Ubbink et al. (1991) as modified by Gilfix et al. (1997) and Pfeiffer et al. (1999). The modification used tris (2-carboxylethyl)-phosphine as a reductant and 7-fluorobenzo-2-oxa-1,3-diazole-4-sulfonate as a fluorescent derivative reagent. Plasma enzyme activities of alanine aminotransferase and aspartate aminotransferase were determined using commercial kits (Zhongsheng Bio-Technology and Science Inc., Beijing, China) on a random access analyzer (Technicon RA 1000, Tarrytown, NY, USA).
Data were analyzed as a completely randomized design by one-way analysis of variance procedure of SAS software (SAS Institute, 2003) with pen used as experimental unit for analysis and the linear and quadratic effects of pyridoxine were also tested by REG procedure of SAS software (SAS Institute, 2003). In our study, the quadratic regression was also used to estimate the supplemental pyridoxine requirement and the requirement was defined as 95% of supplemental pyridoxine level at maximum response.
RESULTS AND DISCUSSION
In our study, the vitamin B6 of basal diets mainly came from wheat and isolated soybean protein (Table 1) and the calculated pyridoxine in basal diets was 1.93 mg/kg. However, the availability of pyridoxine in the basal diets may have been poor and their contribution to meet pyridoxine adequacy may be very limited because the major form of vitamin B6 in plant-source feedstuffs was pyridoxine glucoside and this compounds has poor vitamin B6 bioactivity (Reynolds, 1988; Trumbo et al., 1988). At present, the information on the bioavailability of vitamin B6 in plant-source feedstuffs is limited. In chicks, vitamin B6 in corn and soybean meal has about 40% and 60% bioavailability relative to crystal pyridoxine hydrochlorate salt, respectively (Yen et al., 1976), but the bioavailabilities of vitamin B6 in wheat and isolated soybean protein is unknown in either chicks or ducks. Usually, crystal pyridoxine hydrochlorate salt is assumed to have 100% vitamin B6 activity. Therefore, the duck response to increasing supplemental pyridoxine and the supplemental pyridoxine requirements for ducks are discussed.
In present study, the ducks fed basal diets with no supplementation of pyridoxine had the worst growth performance among all ducks but the weight gain and feed/gain of ducks were improved (p = 0.0027 or p = 0.0277) by increasing supplemental pyridoxine levels (Table 2). This indicated that the basal diet was clearly deficient in vitamin B6 and that the experimental period was adequate to assess the duck response to increasing supplemental pyridoxine. However, only growth depression was observed in vitamin B6-deficient ducks in our study and this was not in agreement with the results of Hegsted and Rao (1945). In their study, severe acute pyridoxine deficiency in young ducklings was also characterized by severe anemia while chronic pyridoxine deficiency in older ducklings produced paralysis, convulsions, severe microcytic anemia, and poor feather development. Unfortunately, anemia was not investigated in our study but the paralysis, convulsions, and poor feather development were not observed in the ducks deficient in this vitamin in present study.
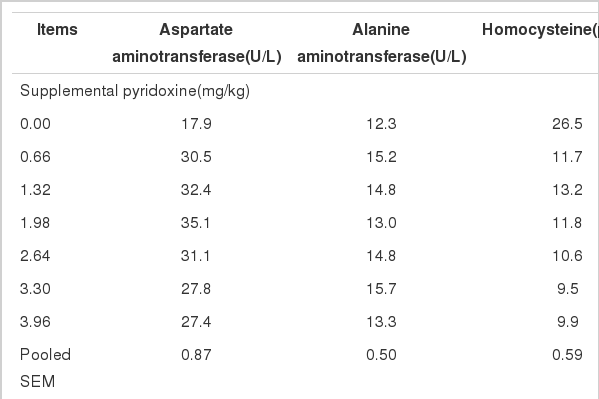
At present, transaminases serve as sensitive indicator for vitamin B6 status. In chicks, the lowering of blood aspartate aminotransferase and alanine aminotransferase activities was found to be specific for vitamin B6 deprivation when the deficiencies of vitamin B6, riboflavin, pantothenic acid, thiamine, niacin, and biotin were tested (Cheney et al., 1965) and similar results were also observed by Yen et al. (1976) and Oloyo (2001). In our study, pyridoxine status influenced aspartate aminotransferase (p<0.0001) but not alanine aminotransferase (p = 0.5219, Table 3). The aspartate aminotransferase activity showed a quadratic response (p = 0.0385) to increasing supplemental pyridoxine levels and the ducks fed vitamin B6-deficient basal diets had the lowest aspartate aminotransferase activity compared with other birds fed pyridoxine-supplemented diets. This was identical with the observed growth response data which showed that the ducks fed basal diets had the lowest weight gain compared with other birds (Table 2). Therefore, our results confirmed the sensitivity of transaminase to pyridoxine deficiency in ducks.
On the other hand, pyridoxine plays a role in the degradation of homocysteine as a coenzyme in the form of PLP and the importance of this vitamin for lowering plasma homocysteine is widely recognized as plasma homocysteine is considered to be a risk factor for cardiovascular disease. Pyridoxine deficiency can lead to the significant elevations of plasma homocysteine in rats or pigs (Smolin et al., 1983; Martinez et al., 2000; Lima et al., 2006; Zhang et al., 2009) and thus plasma homocysteine may be another new indicator for vitamin B6 status, which was also confirmed by our results. In our study, the ducks fed basal diets had the highest level of plasma homocysteine among all ducks and it was reduced linearly (p = 0.0590) or quadratically (p = 0.0538) when pyridoxine was supplemented to basal diets (Table 3). Recently, the reason in which homocysteine increased during prydoxine deficiency was investigated in pigs by Zhang et al. (2009) who found that there was a decrease in PLP-dependent enzymes (cystathionine β-synthase and cystathionine γ-lyase) in the transsulfuration pathway for degradation of homocyteine when this vitamin was deficient.
In the present study, the feed/gain and aspartate aminotransferase showed a quadratic response to increasing supplemental pyridoxine levels (p = 0.0114 and p = 0.0385) and homocysteine showed quadratic trend as this vitamin level increased (p = 0.0538). Therefore, the quadratic regressions were used to estimate the supplemental pyridoxine requirements of ducks (Table 4). According to this regression, the maximum responses for feed/gain, plasma aspartate aminotransferase, and plasma homocysteine were 2.07, 34.0 U/L, and 8.9 μmol/L, respectively, and the corresponding supplemental requirements of this vitamin for these criteria were 2.44, 2.08, and 2.72, respectively (Table 4). The estimated requirements for feed/gain and plasma aspartate aminotransferase were more accurate than the value for plasma homocysteine because they had a much lower p-value and higher R2 for regression compared to plasma homocysteine (Table 4). Furthermore, the estimated responses and requirements for feed/gain and plasma aspartate aminotransferase were close to the observed response data indicating that feed/gain and plasma aspartate aminotransferase were optimized when supplemental pyridoxine levels were 2.64 and 1.98 mg/kg, respectively (Tables 2 and 3), but the observed data was not for the estimated response and requirement for plasma homocysteine. When the pyridoxine concentration of the basal diet (1.93 mg/kg) was included, the total pyridoxine requirements of Pekin ducks were predicted to 4.37 mg/kg for feed/gain and 4.01 for plasma aspartate aminotransferase and these values were much higher than the NRC recommendation (2.5 mg/kg) for White Pekin ducks. However, it should be acknowledged that NRC recommendation for this vitamin was available pyridoxine requirements because this recommendation was obtained from duck trials in which vitamin B6-free purified diets and crystal pyridoxine hydrochlorate salt were used (Hegsted and Rao, 1945).

Supplemental pyridoxine requirements of White Pekin ducklings from hatch to 28 days of age based on the quadratic regression analysis
In conclusion, pyridoxine deficiency can cause growth depression and deficiency of this vitamin can be indicated by decreasing aspartate aminotransferase activity and increasing homocysteine in plasma. The supplemental and total pyridoxine requirements of Pekin ducks from hatch to 28 days of age were 2.44 and 4.37 mg/kg for feed/gain and 2.08 and 4.01 mg/kg for plasma aspartate aminotransferase, respectively.
ACKNOWLEDGMENTS
The work was supported by Beijing Municipal Natural Science Foundation (6133034) and Basic Science Research Program in National Institutes of China (2012cj-4).