Nutritional Characteristics of Forage Grown in South of Benin
Article information
Abstract
In order to provide recommendations on the most useful forage species to smallholder farmers, eleven grass and eleven legume forages grown in Abomey-Calavi in Republic of Benin were investigated for nutritive value (i.e. chemical composition and energy content) and fermentation characteristics (i.e. gas and volatile fatty acid production, organic matter degradability). The in vitro gas production technique was used, incubating the forages for 120 h under anaerobic condition with buffalo rumen fluid. Compared to legume, tropical grass forages showed lower energy (8.07 vs 10.57 MJ/kg dry matter [DM]) and crude protein level (16.10% vs 19.91% DM) and higher cell wall content (neutral detergent fiber: 63.8% vs 40.45% DM), respectively. In grass forages, the chemical composition showed a quite high crude protein content; the in vitro degradability was slightly lower than the range of tropical pasture. The woody legumes were richer in protein and energy and lower in structural carbohydrates than herbaceous plants, however, their in vitro results are influenced by the presence of complex compounds (i.e. tannins). Significant correlations were found between chemical composition and in vitro fermentation characteristics. The in vitro gas production method appears to be a suitable technique for the evaluation of the nutritive value of forages in developing countries.
INTRODUCTION
In Benin agriculture contributes 32.5% to the national total Gross Domestic Product (GDP) (INSAE, 2012) and the livestock sub-sector represents 6.2% of the total GDP (FAO, 2006). Small ruminants are essentially bred by smallholders and this activity generates an important household income during lean periods (Babatoundé et al., 2010). In all Sub-Saharan African area, feed shortages remained the main constraint to ruminant breeding (Adjolohoun et al., 2008; Koura et al., 2015). This is the result of agricultural pressure, urbanization and climate variability on the extensive production system, which now results in poor grazing areas (Koura et al., 2015). According to this author, in some areas of Southern-Benin, the most palatable species such as Panicum maximum and Pennisetum spp. are disappearing. The cropping strategies of herders can no longer resist the increasing changes. The intensification of the production system through forage cultivation is therefore necessary (Adjolohoun et al., 2008) to ensure animal products availability. However, little information is available on the nutritive value of these forages under cultivated conditions that could facilitate their integration in farms. The in vitro gas production technique (IVGPT) has been proposed for several years as a valid method to determine the nutritive value of feedstuffs, since rate and extent of degradation and rumen fermentation can be easily determined by measurement of cumulative gas production (Calabrò et al., 2005a). The IVGPT is to be considered useful especially in developing countries, because the manual system does not require large financial resources and is not a time-consuming method to obtain dynamic descriptions of nutritive value of feedstuffs (Calabrò et al., 2007). Moreover, IVGPT is highly reproducible and allows analyzing many samples simultaneously using small amounts of material. The aim of this investigation was to estimate the nutritive value of some grass and legume forages grown in Abomey-Calavi in Republic of Benin, in order to provide to the smallholder farmers useful recommendations on the best forage species to utilize based upon their nutritional characteristics.
MATERIALS AND METHODS
Study area
The study was carried out on grass grown in the experimental field of the Faculty of Agricultural Sciences of the University of Abomey-Calavi. The University is situated in the Abomey-Calavi common of the Republic of Benin that is located between 6°21′ to 6°42′ North latitude and 2°13′ to 2°25′ East longitude, 11 m.a.s.l. The area has a sub-equatorial climate with two rain seasons alternated with two dry seasons of unequal duration. The rainfall amounts recorded by the Agency for Aerial Navigation Safety in Africa and Madagascar (ASECNA) between 1981 and 2012 are between 739.6 mm and 2,203.3 mm with an average of 1,305.95 mm. The soil is sandy and ferrallitic types. The vegetation consists of shrubs, grassland swamps, swamp forest and mangrove forest on the coastal belt and of semi-deciduous dense forests on bar land area.
Species installation method
The experimental area was parceled into two main blocks, grasses and legumes. These two blocks were separated by a main path of 1.5 m and each block being separated by 1.0 m side aisles; each plot had an area of 5×5 m. Eleven grasses (Andropogon gayanus, Andropogon tectorum, Brachiaria ruziziensis, Cynodon datylon, Echinochloa stagnina, Hyparrhenia diplandra, Panicum maximum var. C1, Panicum maximum var. 673, Panicum maximum (local), Pennisetum purpureum, Vetiveria zizanoïdes) and eleven legumes (Aeschynomene histrix, Cajanus cajan, Centrosema pubescens, Chamaecrista rotundifolia, Gliricedia sepium, Leucaena leucocephala, Moringa oleifera, Mucuna utilis, Stylosanthes hamata, S. scabra, Tephrosia pedicellata) were planted in June 2011. Grasses were installed by bursts of strains, whereas herbaceous legumes either by seed (C. cajan, C pubescens, T. pedicellata, A. histrix, C. rotundifolia, S. hamata, S. scabra, L. leucocephala) or by cuttings (G. sepium, M. oleifera). Some plants (A. histrix, C. rotundifolia, S. hamata, S. scabra) were sown after seed dormancy breaking by hot water. After removing seed dormancy, L. leucocephala was established in plastic bags and later seedlings were transplanted in the field. Planting was done in a continuous line. Spacings were 40 cm (between plants)×40 cm (between lines) for most species. However, it was 80 cm× 80 cm for Pennisetum purpureum and Echinochloa stagnina, 50 cm×50 cm for Mucuna utilis and 1 m×1 m for Cajanus cajan. The sample cuts were made in the upper part of the plants (leaves and stems), during the consolidation phase of forages.
Chemical composition
All the plants were oven-dried for 48 to 72 h at 60°C for dry matter (DM) quantification; the samples were ground to pass a 1 mm screen (Brabender Wiley mill, Brabender OHG, Duisburg, Germany) and analyzed for residual DM, crude protein (CP), ether extract (EE) and ash as suggested by AOAC (2000) procedures (ID number: 2001.12, 978.04, 920.39 and 930.05 for DM, CP, EE, and ash, respectively).
In vitro gas production
The fermentation characteristics and kinetics were studied using the IVGPT, by incubating all the forages at 39°C under anaerobic conditions with buffered rumen fluid (Calabrò et al., 2005b). The test substrates were weighed (1.0005 g±0.0003) in triplicate in 120 mL serum flasks, to which 74 mL of anaerobic medium were added. The rumen fluid was collected in a pre-warmed thermos at a slaughterhouse authorized according to EU legislation (2004) from six male buffalo bulls (Bubalus bubalis) fed a standard diet (neutral detergent fiber [NDF] 45.5% DM and CP 12% DM). The collected material was rapidly transported to the laboratory, where it was pooled, flushed with CO2, filtered through a cheesecloth and added to each flask (10 mL). Three flasks with no substrate were incubated as blanks to correct for the organic matter (OM) disappearance, and gas and end products production.
Gas production of the fermenting cultures was recorded 21 times (at 2 to 24 h intervals) during the period of incubation (120 h) using a manual pressure transducer (Cole and Palmer Instrument Co, Vernon Hills, IL, USA).
The fermentation was stopped at 120 h and the fermentation liquor was analyzed for pH with a pH-meter (model 3030 Alessandrini Instrument glass electrode, Jenway, Dunmow, UK) and sampled for end product analysis. The extent of sample disappearance, expressed as organic matter degradability (dOM, %), was determined by weight difference of the incubated OM and the undegraded filtered (sintered glass crucibles; Schott Duran, Mainz, Germany, porosity # 2) residue burned at 550°C for 5 h. Cumulative volume of gas produced after 120 of incubation was related to incubated OM (OMCV, mL/g) and to degraded OM (Yield, mL/g).
For volatile fatty acids (VFA) determination, fermenting liquors were centrifuged at 12,000 g for 10 min at 4°C (Universal 32R centrifuge, Hettich FurnTech Division DIY, Melle-Neuenkirchen, Germany). One milliliter of supernatant was then mixed with 1 mL of oxalic acid (0.06 mol). Volatile fatty acids were measured by gas chromatography (ThermoQuest 8000top Italia SpA, Rodano, Milan, Italy; fused silica capillary column 30 m, 0.25 mm ID, 0.25 μm film thickness), using external standard solution composed of acetic, propionic, butyric, isobutyric, valeric and isovaleric acids (Zicarelli et al., 2011; Calabrò et al., 2015).
Data processing
The nutritive value of forages was estimated as net energy for lactation (NEl, MJ/kg DM) using the equation proposed by Menke and Steingass (1988):
where, GP is the gas obtained in vitro (mL/200 mg incubated DM) after 24 h of incubation and CP is the content (g/kg DM) of crude protein. For each flask the gas production profiles were fitted to the sigmoid model described by Groot et al. (1996):
where, G is the total gas produced (mL/g of OM) at time t (h), A is the asymptotic gas production (mL/g of OM), B (h) is the time at which one-half of the asymptote is reached, and C is the switching characteristic of the curve. Maximum fermentation rate (Rmax, mL/h) and the time at which it occurred (Tmax, h) were also calculated according to the following formulas (Bauer et al., 2001):
Fermentation characteristics (OMCV, Yield, Dom, and pH) and the model parameters (A, B, C, tmax, Rmax) were subjected, separately for grass and legume, to analysis of variance (PROC GLM, SAS 2000) according to the model:
where, y is the single data, μ is the mean, F is the forage effect (i = 11 for grass and i = 11 for legume) and ɛ the error term. The minimum significant difference (p<0.01 and p<0.05), was used to verify the differences between means using the Tukey test.
The correlation between the chemical parameters and the in vitro fermentation data were also studied (PROC CORR, SAS, 2000) separately for grass and legume samples.
RESULTS
Table 1 and 2 show the chemical composition and the nutritive value of grass and legume forages, respectively. As regards grasses, the local variety of Panicum and Pennisetum purpureum showed a very high CP (25.86% and 27.33% DM) and the lowest structural carbohydrates (NDF: 50.80% and 50.30% DM) content, consequently, energy content (15.14 and 16.10 MJ/kg DM) was very high, though ash level was quite high too (16.42% and 19.68% DM), respectively. On the contrary, V. zizanoïdes showed the lowest CP and energy level (6.69% DM and 2.531 MJ/kg DM, respectively) and the highest NDF (79.66% DM) content. The other forages showed a similar chemical composition, on average equal to: CP 14.7%±3.00% DM, NDF 63.4%±4.41% DM and NEl 6.89±1.89 MJ/kg DM. Observing legume data, M. oleifera was the most interesting forage that had the highest CP, EE, and NEl content (34.45% DM, 6.22% DM and 24.60 MJ/kg DM, respectively) and the lowest NDF (14.03% DM) and acid detergent lignin (ADL) (5.94% DM) contents. Also G. sepium and L. leucocephala showed high values for CP (22.08% and 28.04% DM, respectively) and energy (12.34 and 16.94 MJ/kg DM, respectively) whereas both Stylosanthes spp. showed the lowest CP (12.43% DM) and high structural carbohydrates contents (49.77% DM) and low energy level (5.80 MJ/kg DM). All the other legume forages showed on average the following values: 18.26%±2.02% DM (CP), 46.14%±3.46% DM (NDF) and 8.47±1.57 MJ/kg DM (NEl).
Table 3 and 4 show the in vitro fermentation parameters of grass and legume forages, respectively. Both for grass and legume forages, all parameters were highly (p<0.01) influenced by the forage type. For all forages, data related to gas production (real_OMCV and potential_A) showed similar trend. In grass forages, the average value of OM degradability was 68.41±10.89 and of OMCV 188.45±31.0 mL/g. In particular, in most grass forages (Panicum spp., Pennisetum, Brachiaria, and Echinochloa) dOM values exceeded 70%, whereas Vetiveria showed the lowest dOM value (40.6%; p<0.01). Regarding gas production, the highest value was found in Echinochloa (241 mL/g; p<0.01) and the lowest in Vetiveria (123 mL/g; p<0.01). In legume forages, the average values were 56.48%±12.45% and 155.45%±33.6%, for dOM and OMCV, respectively. For OM degradability, Moringa showed the highest (79.0%; p<0.01) value, and in all the others the values ranged between 27.9% and 64.6%. Regarding gas production, Stylosanthes spp. showed the highest values (195 mL/g; p<0.01). Cajanus showed the lowest (p<0.01) dOM (27.9%) and OMCV (70.0 mL/g) values.
Table 5 and 6 picture pH and VFA produced after 120 hours of incubation for grass and legume forages, respectively. For grass, pH values range between 6.61 and 6.85, whereas for legumes between 6.72 and 6.85. All the parameters reported in both tables, were highly different (p<0.001) between substrates. Grass forage had a higher production of total VFA compared to legume (73.3 vs 59.3 mM/g), however in both families the proportion among the most representative acids are similar (65%, 20%, and 7% for acetic, propionic and butyric, respectively). Regarding grass, the highest value of tVFA was found in Panicum maximum var. 673 (83.91 mM/g) and the lowest in Vetiveria zizanoïdes (53.90 mM/g), although the differences were not statistically significant. In all the cases the acetic acid is the major VFA responsible for this result. Regarding the branched chain fatty acids (BCFA: isobutyrate, isovalerate, valerate), Brachiaria, Panicum maximum local and var. 673, and Pennisetum showed the highest proportion on tVFA (6.53, 6.81, 6.30, and 7.28, respectively).
The legume Stylosanthes scabra had the highest tVFA value (72.5 mM/g) and Cajanus cajan the lowest (35.2 mM/g), although the differences were not statistically significant. Except for Cajanus, where the mean value of BCFA was 6.89.
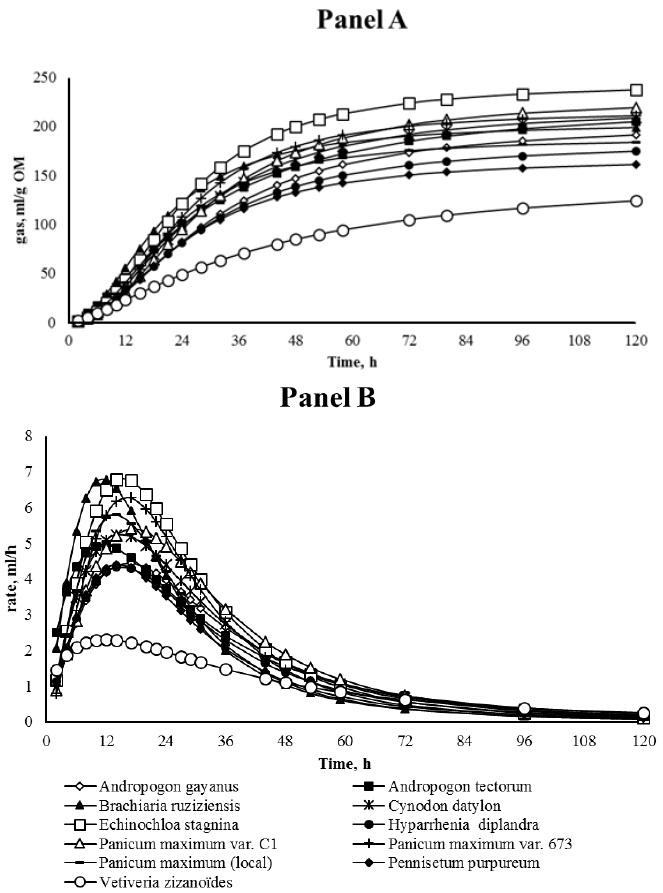
Regarding fermentation kinetics, the parameters presented in Table 3 and 4 (B, C, tmax, Rmax), can be better explained by observing the Figure 1 and 2 (Panel A and B). In particular, for grass forages it is clear that the fermentation process was similar for most of the samples (starts within 6 h of incubation, reaches the maximum around 14 h and finishes gradually at 120 h), except for Vetiveria that showed a very slow process characterized by a fermentation rate profile quite flat (Rmax: 2.31 mL/h and B: 40.0 h; p<0.01). With regard to legume forages, the fermentation profiles were more diversified: Cajanus showed the slowest fermentation process: the gas production curve quite flat and the fermentation rate curve similar to a hyperbole. As an opposite trend, the fermentation for Tephrosia pedicellata and Stylosanthes scabra started quickly and finished quickly (Figure 2, Panel A). Stylosanthes hamata and Moringa oleifera showed a similar shape (Figure 2, Panel B) characterized by very high maximum fermentation rate (7.58 and 7.84 mL/h, respectively). In terms of tmax, Cetrosema pubescens showed the lowest value, whereas Stilosanthes spp. the highest (3.31 vs 7.71 h, respectively; p<0.01). The remaining substrates (Aeschynomene, Chamaecrista, Gliricedia, Mucuna, Thephrosia, and Leuchena) showed a similar profile in terms of time (tmax mean value: 6.01 h) and rate (Rmax mean value: 5.88 mL/h). Table 7 pictures the significance of correlation between some chemical parameters and in vitro fermentation data for grass and legume forages.
DISCUSSION
The chemical composition and in vitro degradability of forages appears quite diversified. Indeed, although the values obtained fall, in part, within those found in the literature (Nasrullah et al., 2003; Calabrò et al., 2007; Adjolohoun et al., 2008; Babatoundé et al., 2011), it is important to mention that not only the variety, but also climatic conditions, sampling site, soil and management conditions and vegetative stage at harvest significantly affect the plant nutrient accumulation (Adjolohoun et al., 2013).
Grass vs legume forages
As reported by Calabrò et al. (2007), compared to legumes, tropical grass forages showed a lower energy level (8.07 vs 10.57 MJ/kg DM), crude protein (16.10 vs 19.91% DM) and a higher cell wall content (NDF: 63.8 vs 40.45% DM), respectively. However, in general, as reported by Calabrò et al. (2007) on Niger forage, the OM degradability is quite low in legume forage compared to grass (mean values: 56.5% vs 68.4%, respectively), probably due to the high lignin content (mean values: 15.21% vs 10.77% DM, respectively) especially in some samples (Cajanus, Chamaecrista, Mucuna, Stylosanthes scabra), associated with the anti-nutritional factors (ANFs) (Adjolohoun et al., 2008). Because of the higher content of carbohydrates in grass than in legume, the total VFA produced is more elevated in the first. The proportion of branched chain fatty acids is higher in legume compared to grass and reflects the protein level in the incubated samples, because these acids derive from the degradation of some amino acids (valine, proline, isoleucine, leucine) (Calabrò et al., 2012). Regarding the kinetics, the greatest part of the fermentation process occurred within the first 60 h for grass and 48 h for legume, to complete slowly in 96 h. In particular, at similar fermentation rates (5.18 and 6.10 mL/h) grass showed a slower fermentation process (tmax: 14.43 vs 6.08 h), for grass and legume respectively, probably due to the higher fermentable carbohydrates content. These results are influenced by the different nature of the substrates used; in particular, all the Graminae were annual or perennial herbaceous species, whereas Leguminosae are shrub, tree or herb.
Grass forage
In the grass forages, the chemical composition is important; in particular, crude protein content was much higher than the critical level of 60 to 80 g/kg DM, below which forage intake is depressed. The in vitro degradability is slightly lower than the range of tropical pasture (50% to 80%) reported by Adjolohoun et al. (2008). There are many studies reporting data on different varieties of Brachiaria, Panicum, Pennisetum and Vetiveria; in particular, Nasrullah et al. (2003) studied the in vitro dry matter digestibility (IVDMD) using the Goering and Van Soest (1970) method for these forages grown as natural pasture, never fertilized, without management intervention and collected in different seasons. The values of chemical composition (CP, EE, ADL) and IVDMD were lower compared to our data, indicating that the water availability and fertilization can improve their nutritive value. On average, the nutritive value of B. ruziziensis is higher compared to the other selected grasses, but the risks of photosensitization and goiter must be emphasized when B. ruziziensis is fed alone to ruminants (Hare et al., 1997). P. maximum (local) shows typical legume characteristics with high protein content (25.9% DM), on the other hand P. maximum var. C1, despite the low crude protein content (10.1% DM), appears more interesting than the two other Panicum spp., due to its high productivity among the perennial grass forages in Southeast of Benin (Adjolohoun et al., 2013). P. purpureum, one of the most productive grass crops in the world (10 to 30 t/ha DM per year), in our trial appears as the best forage in terms of nutritive value (NEl: 16.1 MJ/kg DM) and crude protein content (27.3% DM), even if it is reported that DM yield rapidly declines if fertility is not maintained (Adjolohoun et al., 2008). The fermentation characteristics of V. zizanoïdes are in agreement with the chemical composition, and point out a very slow kinetics and a low OM degradability and VFA production, whose value is similar to that one reported by Nasrullah et al. (2003).
Regarding Andropogon spp., Cynodon and Hyparrhenia few studies were found; in our trial they showed similar chemical characteristics (except for the high lignin content of Cynodon and Hyparrhenia) and the in vitro data indicates a low nutritive value. Echinochloa stagnina, is a perennial semi-aquatic tropical grass; during dry season, it is of outmost importance for maintenance of cattle (Dicko et al., 2003), moreover, it is very productive and highly palatable. In our study, it showed interesting in vitro data in terms of OM degradability, gas and VFA production, and fermentation rate, probably due to the high sugar content.
Legume forage
As a whole, the woody legumes (Cajanus, Gliricedia, Leucaena, Moringa) seem to be richer in protein and energy and lower in structural carbohydrates than herbaceous plants (Aeschynomene, Centrosema, Chamaecrista, Stylosanthes); these characteristics do not always agree with their in vitro data, probably because of the presence of other complex compounds (i.e. tannins). Except for Moringa oleifera, our samples presented lower crude protein and OM degradability values compared to the values reported by Babatoundé et al. (2011) who studied the chemical composition and the in vitro OM degradability, using pepsin and cellulose technique, in legume forages hand-plucked in South Benin during the short dry season. The legume forages showed diversified fermentation kinetics profiles.
M. oleifera, which sample was mainly represented by leaves, was the forage with the highest energy content, due to the high protein and low fiber level; similar data were reported by Babatoundé et al. (2011). It also showed the highest OM degradability, VFA production and fermentation rate, associated with a moderate gas production; this result may in part be explained by the high protein and lipid content (Melesse et al., 2013). In general, low gas production would indicate low degradability in the rumen, but feedstuffs with high protein produce less gas during fermentation. Moreover their extent of degradation is high, because protein fermentation produces ammonia, which influences the carbonate buffer equilibrium neutralizing H+ ions from VFA without release of carbon dioxide. The low gas volume for M. oleifera can be also explained by a high fat content, which contributes to limit fermentation Akinfemi et al. (2009).
On the contrary, the two varieties of Stylosanthes spp. showed the lowest energy content: they were similar in terms of chemical composition and fermentation parameters, even with the higher lignin level and consequent lower NEl value in S. scabra. The high total VFA level was due to the high acetic production, as consequence of the high NDF content.
The low OM degradability, VFA production and very slow fermentation kinetics of C. cajan is due to the highest lignin content registered among all the legume samples; this component, bound to tannins or phenols, is found by many authors (Norton, 1994; Adjolohoun et al., 2008; Babatoundé et al., 2011).
G. sepium and L. leucocephala showed similar fermentation kinetics, with intermediate values in terms of OM degradability and gas and VFA production, associated with a slow process. This result might be due to the presence of high level of tannins in both plants (Shayo and Udén, 1999). Several authors (Norton, 1994; Babatoundé et al., 2011) reported high phenolic and tannin levels in some African woody legumes. In vivo studies evidence that the presence of these ANFs affects the forage nutritive value reducing intake and digestibility (Dzowela and Hove, 1995). In browse species with considerable protein content ANFs interfere with the protein utilization. A low rumen degradation of protein, decreases rumen ammonia concentration; a minimum concentration of ammonia (70 mg N/L) is required from the microbial population in the rumen, lower levels are associated with lower microbial activity and, consequently, lower digestion (Norton, 1994). However, the significance of ANFs becomes more evident when woody foliage is the only feed consumed. The utilization of these species in feeding strategy may be limited, but the interpretation of the protein nutritional value in these species requires information on the nature and actions of tannins, also consideration that they increase aminoacids amount bypassing rumen (Babatoundé et al., 2011).
The other three herbaceous plants (Aeschynomene, Centrosema, Chamaecrista) showed similar chemical composition (i.e. crude protein, structural carbohydrates and energy content) as well as in vitro parameters (i.e. OM degradability, gas production and fermentation rate), but a different VFA profile; in particular, in Centrosema these values were quite low. Among the less-known plants, there are Mucuna utilis and Tephrosia pedicellata; both are more investigated as seeds for ruminants feed, but can also be used as forage (i.e. pasture and hay). Both forages presented quite low nutritive value in terms of protein, lignin, energy, and the fermentation characteristics appear intermediate compared to the other studied legumes.
In vitro gas production
The in vitro gas production method appears to be a suitable technique for the evaluation of the nutritive value of forages in developing countries (Calabrò et al., 2007; Babatoundé et al., 2011) where financial resources are limited. According to Calabrò et al. (2007), this method gives an assessment of the degradability of both soluble and insoluble fractions of forages and useful information about the fermentation kinetics, and final products (i.e. VFA). In this study, pH value at the end of the incubation, both for grass and legume forages, was adequate for cellulolytic activity, indicating the efficiency of the buffer in the in vitro system. In general, the final gas production recorded (OMCV) was similar to the potential value estimated by the adjusted model (A) indicating that the incubation time (120 h) is adequate to complete the fermentation process of these kind of substrates. The ratio of substrate truly degraded to gas volume (partitioning factor, PF) in our trial ranges for all substrates from 3.07 to 4.94 mg/mL, and falls within the range for conventional feed roughages (PF: 2.74 to 4.65 mg/mL) reported by Getachew et al. (1998). The escape of tannins from the feed during fermentation, contributes to the DM loss but does not influence the gas produced; so our result indicates that the potential tannin content of the tested forages did not affect the in vitro OM fermentation. The VFA produced during the incubation gives information about the energy released by carbohydrates during fermentation and directly available to the animal. All samples showed moderate levels of total VFA and the proportion among the singular acids, reflects that one produced in the rumen with mixed forage/concentrate ratio. As expected, both in grass and legume, tVFA data reflect the trend of gas production (OMCV) and OM degradability (coefficient of correlation 0.80 and 0.89, p<0.01, respectively) indicating that all the degraded OM fermenting gives energy.
According to Bulgden et al. (2001), the in vitro fermentation characteristics follow the trend of their chemical composition. The analysis of correlations between some IVGPT parameters and some chemical data confirmed the influence of certain features on the fermentation process. In particular, for grass forages OM degradability and gas production (Yield) are significantly (p<0.05) correlated with some chemical characteristics (protein, EE, energy and ash); as expected, the structural carbohydrates negatively influenced OM degradability and fermentation rate (correlation coefficient: −0.76 p<0.01) (Calabrò et al., 2007). In legume forages, less significant correlations were found: in particular, kinetic parameters are not affected by chemical data, whereas in vitro some parameters where negatively correlated: OM degradability with cell wall (NDF: −0.62, p<0.05; ADL: −0.90, p<0.01), tVFA with ADL (−0.76; p<0.01) and gas production (Yield) resulted correlated with crude protein (−0.87; p<0.01), EE (−0.60; p<0.05) and ash −0.40; p<0.05). These nutrients can negatively interfere with the microbial activity, as reported by Calabrò et al. (2007).
CONCLUSION
Our evaluation of cultivable grasses and legumes in Benin revealed remarkable characteristics for their chemical composition, nutritive value and Dom. Several authors worked on these species, but mainly in natural than artificial pasture. Some data obtained in this investigation confirm that already present in the literature (Brachiaria, Panicum, Pennisetum, Cajanus, Gliricedia, Leucaena, Moringa); other results are extremely interesting because they are related to less studied plants (Andropogon, Cynodon, Echinocloa, Hyparrhenia, Vetiveria, Aechynomene, Mucuna, Stylosanthes, Tephrosia).
The in vitro method utilized was helpful to obtain the nutritive value and describe the fermentation kinetics of most common cultivated legume and grass forages from Benin. However, the complexity of some constituents present in tropical plants (i.e. lignin and secondary compounds), influence the in vitro fermentation. The information reported could help to better supplement the animal diet based on poor quality forage and assist the small ruminant breeds in increasing their productivity using cultivated forage in South Benin and in other regions of West Africa.
ACKNOWLEDGMENTS
The authors want to thank Dr. Maria Ferrara for her technical support in chromatographic analysis at the Department of Veterinary Medicine and Animal Production, University of Napoli Federico II, Italy. The study was supported by “Benin Buffalo Breeding Project” (BBB-Project) 2014, financed by University Federico II, Napoli (Italy); Responsible Professor Bianca Gasparrini.
Notes
CONFLICT OF INTEREST
We certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.