Screening of BCL-2 associated X protein gene polymorphism associated with scrotal hernia in domesticated swine using polymerase chain reaction-restriction fragment length polymorphism
Article information
Abstract
Objective
This study was conducted to screen scrotal hernia in domesticated swine from selected breeders in the Philippines. This defect is associated with a cytosine to thymine mutation in the BCL-2 associated X protein (BAX) gene of swine.
Methods
Genetic screening was done by DNA extraction followed by amplification and digestion using polymerase chain reaction-restriction fragment length polymorphism, amplifying the 416 bp region of the BAX gene that was subjected to digestion using the Ear I enzyme. Sequencing was also conducted to validate the results.
Results
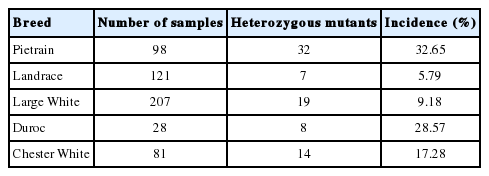
Results revealed that out of 538 samples tested, 411 (76.4%) of the samples were found to be normal whereas the remaining were carriers of the mutation in which 80 (14.9%) were heterozygous mutants and 47 (8.7%) were homozygous mutants. Pietrain breed was found to have the highest incidence.
Conclusion
Having a scrotal hernia eliminates the chances of using the boar as a breeder stock because the following generations arising from it would most likely exhibit herniation. It is therefore advised to establish a genetic screening method for Scrotal Hernia in the Philippines to eliminate the negative gene from the herd.
INTRODUCTION
Swine industry in the Philippines is the second largest contributor to the agricultural sector, coming in next to rice, contributing 12 percent to the country’s total value of agricultural production. In 2011, swine population inventory was 12.3 million head, that gave way to the production of approximately 1,940 thousand metric tons of hog live weight in the same year [1]. The swine industry is believed to be one of the most organized and highest adopter of technologies as compared to other local animal industries in the Philippines. However, overall swine farm production efficiency in the country still lags behind other countries due to poor reproductive performance. Tonnage of live weight produced per sow per year was seen to be 500 kg lower compared to other swine producing countries in 2011. Application of gene markers that are associated to economically important traits offers great potential in improving the breeding selection process. Moreover, gene marker assisted selection can also facilitate genetic improvement in terms of carcass quality, disease resistance and in screening against genetic defects in swine breeding herds.
Scrotal Hernia is one of the most common defects in swine, having a prevalence between 0% to 1.5% in different breeds [2] with a heritability of 0.2 to 0.6 [3]. Scrotal hernia is strictly found in males and is characterized by the protrusion of internal organs, such as the intestines, to the scrotum. This affects their efficacy in feeding, thus altering their capacity to grow. Moreover, these individuals would require higher feed quality plus additional health care considerations increasing the losses for the breeder. Having this defect eliminates the chances of using the boar as a breeder stock since the following generations arising from it would most likely exhibit herniation. In severe cases, the intestine may experience blockage of blood flow, resulting to a case known as strangulated hernia which can rapidly lead to death.
Two causes have been formulated leading to the formation of hernia in swine. This may be due to the failure of the processus vaginalis (PV) to obliterate after the descent of the testis, or the failure of the internal inguinal ring to close, thus resulting to the contents to exit through the external inguinal ring [4]. Treatment of this defect is very sensitive as it may result to the falling out of the intestines, especially during castration.
Manifestations of hernia may arise during weaning ages. The most prominent expression of this defect is a bulge located near the groin area. Studies show that this defect is influenced by many candidate genes, hence being polygenic, all of which are involved in the process of testicular descent and scrotal development. Several loci have been determined in various studies involving single nucleotide polymorphisms (SNPs) or single nucleotide polymorphisms [5–8]. Several chromosomal regions have also been identified to be significantly contributing to scrotal hernia, particularly, SW1686 and SW2157 on SSC2 and SW0075 on SSC13 [9,10].
A particular gene associated to the formation of scrotal hernia was of interest in this study, the B-cell lymphoma 2 (BCL2)- associated X protein or the BAX and its corresponding SNP [3]. Tanyel [11] illustrated the possible pathway of PV obliteration in their study in 2004 where it was stated that BAX was involved in the initiation of the cascade of programmed cell death, leading to the obliteration of the PV [10,12] Limited studies have been conducted regarding this defect; however, there are a number of candidate genes found to be associated with it. On the contrary, the said gene still requires further studying and testing. Findings of Gatphayak et al. [3] revealed that the BAX gene has a direct association with the Scrotal Hernia defect. The BAX gene is accessible at National Center for Biotechnology Information (NCBI) having a GenBank Accession # NC_010448.3.
Environmental and genetic factors were also seen as contributing factors to the formation of Scrotal Hernia. However, the influences of these have not been fully established up to present. Studies being recorded as early as 1941 have been concerned about this defect. No restrictions to any breed have been noted, but its development was estimated to be 0.29 for Duroc in 5,711 Duroc-sired male pigs, and 0.34 for both Landrace and Yorkshire-sired in 2,227 Landrace-sired and Yorkshire-sired male pigs, respectively [2]. Some studies indicate the possible mode of inheritance of this defect as incomplete dominance, however, very little proof has been generated to establish its viability. Recommendations suggest the elimination of the boar which produces a litter exhibiting high rate of scrotal herniation. Those which produce litters which show minimal rates of herniation do not necessarily suggest that the boar is a transmitter [13].
A reported frequency of 2% in the entire population of swine manifested the presence of scrotal hernia in Germany. Thai swine breeders, on the other hand, have seen this as an economic problem with about 1% of the population in industrial swine farms and 5% in small-sized farms exhibiting the manifestation of the said defect [3]. However, there have been no prevalence studies conducted in the Philippines about this defect at present.
The polymerase chain reaction- restriction fragment length polymorphism (PCR-RFLP) or cleaved amplified polymorphic sequence (CAPS) is a less expensive method capable of locating genes associated with certain genetic disorders [14]. Mutations such as single nucleotide polymorphism, multiple nucleotide polymorphism, deletion, duplication and combination often cause the formation or elimination of a restriction enzyme recognition site. The method uses this circumstance as an advantage to analyze the DNA.
This study was conducted to screen the mutation in BAX gene associated with scrotal hernia, determine its frequency in a population of domesticated swine and identify incidence in different breeds tested. A further aim was to establish a genetic screening method for Scrotal Hernia in the Philippines.
MATERIALS AND METHODS
All applicable institutional guidelines for the care and use of animals were followed.
Sample collection
Five milliliters (5 mL) of blood were collected from 538 randomly chosen swine (207 Large White, 121 Landrace, 81 Chester White, 28 Duroc, and 98 Pietrain) from selected commercial breeders all over the Philippines. Extracted blood samples were placed in vacutainer tubes containing ethylenediaminetetraacetic acid (EDTA) buffer. These tubes were then stored in a cooler maintaining a temperature of less than 4°C prior to transportation to the laboratory for DNA extraction and analysis.
For the Scrotal Hernia positive control, tissue samples from two herniated pigs were obtained through the castration of the affected swine. The samples were, likewise, stored in a cooler prior to its transportation.
DNA extraction
The DNA from each collected blood sample was extracted using the Wizard Blood Extraction Kit (Promega Corp., Madison, WI, USA) following the manufacturer’s instructions. Conversely, DNA from the tissue of the herniated swine was extracted using the Qiagen DNAeasy Blood and Tissue Kit (Qiagen Inc., Valencia, CA, USA).
Optimization
This method was used since it allowed the determination of the optimum annealing temperatures at which the oligonucleotide primers would work best [15]. Annealing temperature and cocktail mix optimization procedures were first done prior to the amplification of the extracted DNA samples. Using gradient PCR, several temperatures were tested (51°C to 68°C) as to determine the most ideal annealing temperature for the amplification of the genes of interest.
Polymerase chain reaction- restriction fragment length polymorphism
After optimization, DNA samples for Scrotal Hernia were subjected to thermal cycling to amplify the 416-bp region of the BAX gene. A cocktail mixture was prepared with the following components: 2 μL of 5× PCR buffer, 1 μL of 25 mM MgCl2, 0.5 μL each of dNTPs and primers, 0.1 μL of Taq polymerase adjusted to a final volume of 10 μL using dH2O.
The samples were amplified under the following conditions: initial incubation for 4 minutes under 94°C, followed by denaturation at 94°C with 35 cycles for 45 s, annealing temperature of 63°C for 1 minute, elongation of 45 s at 72°C, root elongation of 5 minutes at 72°C and holding temperature of −4°C. The primers used, synthesized by NEB Inc. (Ipswich, MA, USA), were adapted from the study of Gatphayak et al [3] (Table 1).
To detect the SNPC119T of the BAX gene, RFLP mixture for each sample was prepared containing 1.75 μL of high performance liquid chromatography water, 0.5 μL of 10×Tris-acetate-EDTA buffer and 0.25 μL of the EarI restriction enzyme, manufactured by NEB Inc. (Ipswich, MA, USA). Two and a half μL of the PCR products were then added to each RFLP mixtures and incubated for 30 minutes at 37°C. Resulting products were loaded into 3% agarose gel using 5 μL for each sample, and 5 μL of the 1 kb+ hyperladder (Invitrogen, Carlsbad, CA, USA). Gel electrophoresis subsequently followed where 3% agarose gel was utilized as the medium for this procedure. Gel red was used for the visualization of the amplicons under the Protein simple UV Gel Documentation System (San Jose, CA, USA). Positive controls of Scrotal Hernia were sent to First Base Services in Malaysia for sequencing. Results from DNA sequencing were used to verify the results of the RFLP tests.
RESULTS AND DISCUSSION
Results presented an optimal temperature of 68°C due to the production of clearer bands without smearing as well as the absence of non-specific amplicons in the gel. After optimization, the PCR results revealed successful amplification of the BAX gene having fragment size 416 bp.
BAX gene has been studied in several researches, however, most were associated with man and other organisms, with limited investigation of its effects and roles in swine [16]. It has been sufficiently proven that BAX plays the same roles several mammalian groups, which may indicate similar roles for BAX in swine as well. This gene was found to be associated with apoptosis, functioning contrary to the BCL2 which was found to inhibit cellular death. Despite the differences in their effects, the members of the BAX family exhibit similar sequences to that of BCL2. Along with two other pro-apoptotic protein families, the BH3 family and the BCL-2 homologues antagonist/killer (BAK) family, these work hand in hand in initiating apoptosis wherein BH3-only families act as damage sensors and instigate antagonistic action to that of the anti-apoptotic proteins, and the BAK and BAX families induce cellular death [17]. The BAX gene possesses a hydrophobic carboxyl terminal which targets the mitochondria resulting in a breach in the integrity of mitochondria, allowing the exit of pro-apoptotic proteins such as cytochrome C [18].
Restriction fragment length polymorphism
The restriction site of the Ear I restriction enzyme is the 5′...CTCTTC...3′ region of the amplified segment which cuts it into two segments of approximately 296 bp and 120 bp. Failure of the restriction enzyme to recognize this restriction site is attributed to the SNPC119T as stated in the study conducted by Gatphayak et al [3]. Mutations in the restriction site, on the other hand, cause the inability of the restriction enzyme to cut the segment due to the absence of a cutting site. Heterozygous mutants would have a normal strand and a mutated strand. The RFLP assay for heterozygous mutants would result in three fragments consisting of fragments with 416 bp, 296 bp, and 120 bp. Homozygous mutants will only have 1 band as they have no Ear I cutting sites.
RFLP results for samples tested for Scrotal Hernia can be seen in Figure 1. Normal samples showed two bands having sizes of 296 bp and 120 bp. While lanes that have the presence of the 416 bp, 296 bp, and 120 bp bands indicate carriers. Samples with only the 416 bp band imply that the sample is from a homozygous mutant.
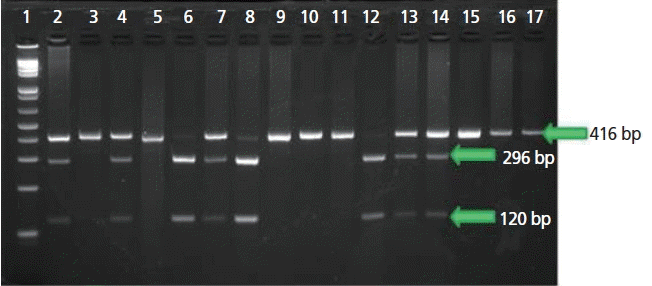
Agarose gel electrophoresis of polymerase chain reaction-restriction fragment length polymorphism test for the BCL2-associated X protein (BAX) gene. Lane 1 to 100 bp molecular weight ladder. Lanes 2, 4, 13, and 14 are classified as Carrier with 416, 296, and 120 bp; Lanes 3, 5, 9, 10, 11, and 16 are classified as mutant with 416 bp undigested band; Lanes 6, 8, and 12 are normal with 296 and 120 bp. Lane 17 is the positive control used.
Positive controls were also tested to confirm the ability of Ear I to recognize the cutting site, and to serve as the basis of comparison for the bands produced by the other samples. It can be seen that the positive control has only one band which follows and agrees with the results of Gatphayak et al [3] who stated that the mutation would render the said restriction enzyme inactive due to SNPC119T. Furthermore, results for the sequence of the Scrotal Hernia positive controls confirmed the Cytosine to Thymine mutation at the BAX gene (Figure 2). Cleaned sequences obtained from Mega6 software were then aligned, and assembled using Vector NTI Advanced 11.5 software. The assemblage was then inputted into the basic local alignment search tool in NCBI as a confirmatory test to assure that we were able to amplify the correct regions, that is, the BAX gene. Results show that a 99% to 100% identity was obtained for the scrotal hernia samples to the Sus scrofa BAX gene (NC_010448.3).

DNA Alignment of the positive controls of scrotal hernia showing the cytosine to thymine mutation at the 119th base of the BCL2-associated X protein (BAX) gene.
Out of the 538 amplified samples, it was found that only 76.4% were normal. The remaining 23.6% has the mutation in BAX gene in which 14.9% were heterozygous mutants and 8.7% were homozygous mutants. The results present a high frequency of mutation in the tested population. According to Gatphayak et al [3], while the amplified region is located on the intron of the BAX gene, it can still have a possible effect on the splicing process and these alterations in the splicing can lead to the defect. However, further study of the biological role of the BAX gene is essential to fully understand the mechanism of how the mutation directly contributes to the development of hernia. Other SNP in the regulatory and coding region in the BAX gene can also be studied.
There are no studies yet showing any breed correlations in the development of scrotal hernia. However, this study showed that the Pietrain breed showed highest incidence of the mutation, out of 98 Pietrain samples tested, 26 (26.5%) were found to be homozygous mutants while 32 samples were heterozygous mutants. This was followed by Duroc with 4 homozygous mutants and 8 heterozygous mutants out of 28 (14.29%), then followed by the Chester White, Large White, then Landrace (Tables 2 and 3).
According to Magee et al [19], scrotal hernia heritability was estimated to be 0.157. Although various evidence points to the polygenic nature of scrotal hernia, there is still no definite evidence supporting it up to present. Moreover, environmental factors are also considered as contributors to the development of scrotal hernia [8]. At present, the only way to reduce the effect of this defect is for the breeders to eliminate boars which produce litter exhibiting high rate of scrotal herniation [13]. Still, this method does not consider the influence of sow. Thus, screening for the mutation in BAX gene associated with scrotal hernia may help reduce the chances developing the defect as not only boars will be tested but also the sows that may be carrying the defect.
IMPLICATIONS
This study entailed the use of 538 randomly selected breeder swine in order to detect the presence of the polymorphism in BAX gene associated with Scrotal Hernia in the commercial swine population. The data showed that 76.4% of the tested population were free of the mutation in the BAX gene. The remaining 8.7% and 14.9% were homozygous mutants and heterozygous mutants, respectively. This indicates a high prevalence and frequency of the mutation in the tested population from the swine industry in the Philippines. Thus, this may be an indication of the high incidence of this defect in the swine populations in the country, showing the importance of conducting genetic screening methods to eliminate breeders that are carriers of the defect.
ACKNOWLEDGMENTS
We thank Dr Arnel N Del Barrio, Philippine Carabao Center (PCC) Executive Director, for the support to finish the study. Thanks to the Department of Science and Technology-Philippine Council for Agriculture, Aquatic and Natural Resources Research and Development (DOST-PCAARRD) for the research grant under the Swine Genomics Project 2 and for monitoring the progress of this research. Also, special thanks to all the staff of the Animal Health Unit of PCC for their technical support.
Notes
AUTHOR CONTRIBUTIONS
JG Manalysay and CN Mingala designed the experiment, analyzed and interpreted the data, and did the critical and final revision of the manuscript. While ND Antonio, RLR Apilado, JF Bambico did the acquisition of data and drafting of manuscript.
CONFLICT OF INTEREST
We certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.