Effects of conjugated linoleic acid on the performance of laying hens, lipid composition of egg yolk, egg flavor, and serum components
Article information
Abstract
Objective
This experiment investigated the effects of dietary supplementation with conjugated linoleic acid (CLA) on the serum components, laying hen productivity, lipid composition of egg yolk, egg flavor and egg quality.
Methods
Healthy 28-week-old Hy-Line white laying hens (n = 480) were divided randomly into 4 groups, 6 replicates/group, 20 birds/replicate. The 30-day experimental diets included 0% (control), 0.4%, 0.8%, and 1.6% CLA. Some serum indices of the birds, and egg production, quality, fatty acid composition, egg quality were measured.
Results
The dietary supplementation with 0.4%, 0.8%, and 1.6% CLA did not significantly affect the laying rate and feed intake, as well as calcium ion and phosphorus ion concentration in serum (p>0.05). However, the CLA had significantly increased the strength of eggshell, decreased the odor, flavor, and taste of egg yolk, deepened the color of egg yolk, increased saturated fatty acids and polyunsaturated fatty acids, and reduced the monounsaturated fatty acids (p<0.05). On the other hand, the dietary supplementation with 1.6% CLA had significant effects on feed/gain, and improved serum hormones. Dietary supplementation with 0.4% and 0.8% CLA can significantly enhance the activity of alkaline phosphates.
Conclusion
CLA has no effect on production performance, but does enhance the lipid content of the egg yolk and the strength of the eggshell.
INTRODUCTION
In China, people’s living standards have greatly improved with the rapid development of the economy, and the people’s diets have changed accordingly. In recent years, the increased consumption of high-fat and high-cholesterol foods such as meat and eggs has resulted in higher incidences of cardiovascular disease, including in young people [1]. This has become a major public health problem. Cardiovascular diseases not only seriously harm people’s health, but also consume a large amount of medical resources, according to estimates of the World Health Organization[2]. If no effective measures are taken, this situation will cause huge economic losses for China in the next 10 years [2]. Medicinal drugs and alterations in life style are effective ways to control these diseases. However, the long-term use of drugs carries risks of adverse side effects, and it is difficult to change the eating habits of a large population.
A relatively new concept in disease prevention is the consumption of functional foods, that is, foods with added nutritional or health-promoting benefits. The potential additive considered here is conjugated linoleic acid (CLA), discovered in the 1980s [3]. Since its discovery, investigations have shown that CLA may benefit immune modulation, weight reduction, and prevention of diseases such as cancer and arteriosclerosis [4–6]. Thus, CLA has attracted wide attention in the fields of medicine, food science, and nutrition.
In animal nutrition, a main goal is to produce livestock and poultry products that contain CLA, while maintaining flavor and other desirable food qualities. Thus, people can enjoy the food while preventing cardiovascular disease. Research has proved that CLA can be enriched in meat [7] and eggs [8,9]. For the meat industry, CLA has been shown to enhance the immunity of animals [10,11], reduce the carcass fat content of pigs [12–14], and improve the quality of meat [14,15]. However, the effect of CLA on the egg production performance and egg quality of laying hens is controversial.
Jones et al [9] reported that the rate of egg production was significantly decreased by CLA in hens’ diets, even at levels as low as 0.5% to 1.0%. Moreover, in most studies with poultry, CLA supplementation led to reduced feed intake [8,16], although Reas [17] showed that CLA did not change feed intake at levels of 1.0%. Shang et al [18] found that dietary CLA negatively correlated with feed consumption, body weight gain, rate of egg production, egg weight, and feed/gain. However, Cherian et al [19] reported that CLA-supplementation did not affect feed consumption, daily egg production, feed efficiency, or egg weight. Another study reported a 7% reduction in egg production, but no reduction in egg or yolk weight, after a 68-wk-long trial in which CLA was included at 0.5 and 1.0 g/kg in the hens’ diet [9]. Yet, Schafer et al [20] reported that feeding 29 g CLA/kg for 80 wk did not affect egg production or egg weight, and another investigation found that the performance of laying hens was not affected by dietary CLA concentration [21].
Regarding egg quality for the consumer, Ahn et al [16] reported that dietary CLA increased the firmness of hard-cooked egg yolk, making the yolks rubbery and elastic, which may reduce their acceptability. Shang et al [18] reported that the addition of CLA to more than 4% of diets had a negative effect on egg yolk flavor, but when the amount was less than 3%, no adverse effect was observed.
In an attempt to resolve these controversies, we investigated the effects of dietary CLA on performance, egg quality, and serum components of laying hens. The findings are expected to benefit the successful development of CLA-enriched eggs.
MATERIALS AND METHODS
Birds, diets, housing, and sampling
Four hundred and eighty Hy-line white hens at 28 weeks of age were included in the study. Hens were randomly divided into 4 groups of 480 birds. Each group was further randomly divided into 6 replicates of 20 hens.
In each replicate, hens were housed in wire-floored metal cages (40 cm wide×45 cm long×45 cm high), 4 hens per cage. Hens were provided with food and water ad libitum during the 30-d experimental period. The photoperiod was set at 17 h light-to-7 h dark. The room temperature was maintained at 25°C±5°C.
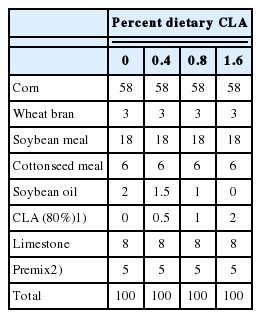
Four corn-soybean meal-based diets were formulated using 0%, 0.5%, 1%, or 2% (w/w) of a lipid product that contains 80% CLA (Aohai, Qingdao, Shangdong Province, China), resulting in diets containing 0%, 0.4%, 0.8%, and 1.6% CLA, respectively (Table 1). Soybean oil was used (1.6%, 1.2%, 0.8%, and 0% w/w, respectively, of the total diet) to supplement the amount of CLA used, to equalize the energy intake in the diets. To avoid fat oxidation, diets were freshly prepared each week, and 0.04 kg/1,000 kg of an antioxidant (0.02% ethoxyquin, Shanghai San Wei Feed Additive, Beijing, China) was added to all diets. The diets were formulated to provide 2,700 kcal metabolisable energy/kg, 0.98% total lysine, 0.47% total methionine, 3.52% calcium, and 0.61% total phosphorus. The addition of vitamins and trace elements conformed to the provisions of the 1,224 announcement by the Ministry of Agriculture of China (2009).
Egg production and egg weight were recorded daily, and feed consumed was recorded weekly for 30 days. Average feed efficiency was calculated. Four eggs from each replicate for each treatment were used to measure the quality of the main egg components (shell, yolk, and albumen) on day 27. Yolk color was determined by comparing yolk color to the Roche color fan.
On day 30, 12 hens (2 birds per replicate) were randomly selected from each group to collect peripheral blood from the wing vein. Serum was separated after blood coagulation, and stored at −20°C for subsequent analysis.
At days 7, 14, 21, and 28, four eggs were randomly selected from each replicate (24 eggs per treatment) and separated into yolk and albumen to analyze the fatty acid composition. Chamruspollert and Sell (1999) reported that the maximum level of CLA in egg yolk lipids appears from 10 to 11 d after CLA feeding is initiated. Therefore, using the ISO standard 5492 (2010) for sensory analysis, ten eggs were randomly selected in each treatment to assess the sensory indexes of egg yolk at 11 d.
Laboratory analysis
The fatty acid composition of egg yolk was analyzed in accordance with the method described by Raes et al [17]. Heptadecaenoic acid (17:0) was used as an internal standard. Fatty acid methyl esters were analyzed by gas chromatography on an HP6890 GC system (Hewlett-Packard, Wilmington, DE, USA) installed with a Chrompack capillary column (CP-Sil 88 column, 100 m×250 μm×0.25 μm, Varian, Palo Alto, CA, USA). The chromatography conditions were as follows: injector temperature at 250°C; detector temperature at 250°C; helium as the carrier gas, split ratio 1:40; temperature program set to 180°C for 45 min, followed by an increase of 10°C/min to 215°C, and then maintained for 17 min. Peaks were identified compared to the retention times of the corresponding standards (Sigma, St. Louis, MO, USA; Matreya Biochemicals, State College, PA, USA). The peaks identified included fatty acids between 14:0 and 24:1 and 6 different CLA isomers. The trans-8, cis-10 CLA isomer was probably present in the CLA product, but its peak overlapped with that of cis-9, trans-11 CLA and it could not be differentiated. The determination was carried out at the China Ministry of Agriculture Feed Industry Center.
Egg quality was measured using an egg quality analyzer (EMT-5200 Robotmation, Japan). Calcium (Ca2+) was measured by the methylthymol blue colorimetric method; phosphorous (P5+) was determined by the ultraviolet endpoint assay. The activity of alkaline phosphatase was assayed by a microtiter assay (with para-nitrophenyl phosphate as the substrate). All indexes were measured using assay kits (Beijing Lidman Biological Technology, China) in accordance with manufacturer’s instructions.
The hormone content of sera was measured using a chemiluminescent immunoassay at General Hospital of Jinan Area Military.
For evaluation of the sensory indexes, 12 eggs were taken from each treatment, stored at room temperature for 7 days, boiled in water for 15 minutes, and evaluated by a panel of experts from the Food Science College at Jinan University. The color, smell, taste, and mouth feeling of the egg yolks were scored in accordance with the standard of sensory evaluation of egg yolk (Table 2).
Statistical analyses
All data are presented as mean±standard error of the mean, and analyzed using ANOVA analysis of variance with the statistical software SPSS 11.0 (SPSS, Chicago, IL, USA), followed by the Duncan multiple range test. Less than 0.05 p value was considered a significant difference. The effects of increasing CLA were partitioned into linear and quadratic components using polynomial contrasts
RESULTS
Production performance
Average daily feed intakes were similar in all treatment groups, while hens fed the 1.6% CLA diet tended to consume slightly less feed than other hens (Table 3). Hens fed the 0.4%, 0.8%, and 1.6% CLA diets produced more eggs than the hens fed with the control diet (0% CLA), although the differences in all treatment groups were not significant. However, dietary supplementation with 1.6% CLA had significant effects on feed conversion (p<0.05; Table 3).
Egg quality
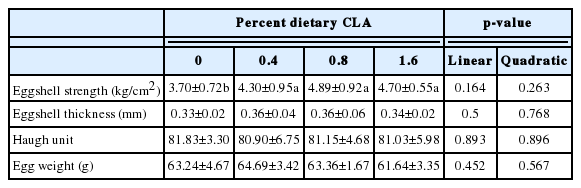
Dietary supplementation with CLA, at all concentrations, was associated with significantly stronger eggshells (p< 0.05; Table 4). Eggshell thickness, the Haugh unit, and egg weight were similar among the different treatment groups (Table 4).
Sensory parameters of egg yolk
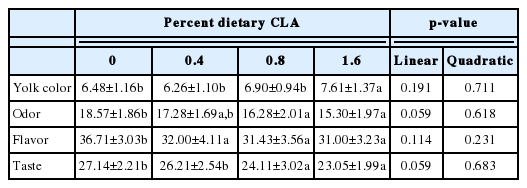
Increasing CLA concentrations were associated with significant reductions in consumer desirability of egg yolks, in terms of odor, flavor, and taste (p<0.05, all; Table 5), and deepening of egg yolk color (p<0.05).
Fatty acid profile of egg yolk
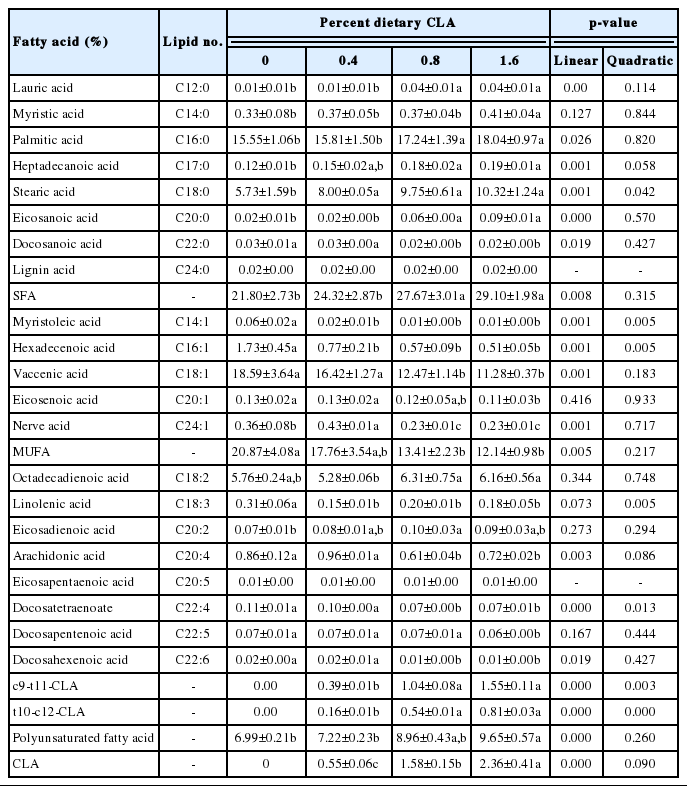
CLA supplementation was associated with significantly higher total saturated fatty acid (SFA) and polyunsaturated fatty acid (PUFA) contents compared with the control group (p<0.05), and significantly lower monounsaturated fatty acids (MUFAs; p<0.05; Table 6).
Compared with the control group, almost all the SFAs in yolk were significantly altered in the groups given dietary CLA, except for lignin acid. Hens fed 1.6% CLA had significantly higher lauric acid, myristic acid, palmitic acid, heptadecanoic acid, stearic acid, and arachidic acid in egg yolk compared with the controls, while the content of behenic acid content was significantly less.
Except for eicosapentaenoic acid, all the MUFAs in egg yolk were altered with increasing dietary CLA supplementation, relative to the controls; the contents of octadecadienoic acid and eicosadienoic acid were significantly higher, while that of linolenic acid, arachidonic acid, docosatetraenoate, docosapentenoic acid, and docosahexaenoic acid were significantly lower (p<0.05, all).
The total amounts of CLA transferred into egg yolk lipids correlated significantly and positively with dietary CLA (p<0.05).
Hen serum components
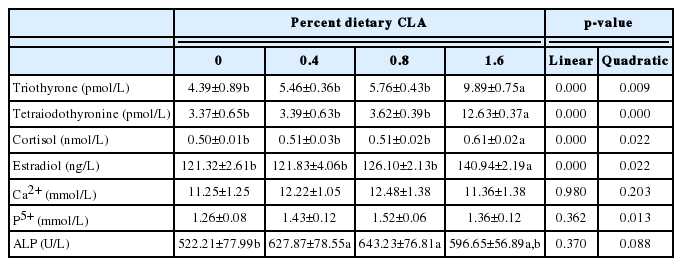
Increases in dietary CLA were associated with tendencies toward increases in triothyrone, tetraiodothyronine, cortisol, and estradiol (Table 7). The levels of serum hormones were significantly higher in hens fed diets containing 1.6% CLA than in the other hens (p<0.05). Analysis showed that CLA was associated with significantly enhanced alkaline phosphatase enzyme activity (p<0.05); the concentrations of calcium and phosphorus ions tended to increase although not significantly.
DISCUSSION
Production performance
It has been reported that reductions in feed intake, egg production, egg weight, and feed conversion are associated with increasing CLA concentrations in diets [8,16,18,22]. Our study showed that the laying rate of hens fed with diets containing CLA was higher than that of the controls, while the average daily feed intake remained similar among all treatments. When hens were fed diets containing 0.4% or 0.8% CLA, their egg-laying and feed conversion rates were higher than those of the control group. However, in hens fed 1.6% CLA, these rates were lower than the control group. These results consistent with that of previous works [18]. Estrogen has been shown to promote follicular growth, while cortisol reduces egg production [23]. In this study, when the proportion of dietary CLA was 0.4% or 0.8%, the levels of serum thyroid hormone and estradiol were higher than that of the control group, resulting in better production performance as compared to hens without CLA supplementation [24,25]. However, when the hens were fed with a diet containing 1.6% CLA, the feed consumption decreased slightly. This might be due to the negative effect of cortisol (which was significantly higher in the 1.6% group, Table 7) that offsets the positive effect of thyroid hormone and estradiol [24,25].
Egg quality and flavor of egg yolk
Compared with the hens given the non-CLA control diet, the eggshell of the hens fed some proportion of CLA were significantly strong. This suggests that CLA could improve concentrations of alkaline phosphatase and estradiol in laying hens, which might enhance the hydrolysis of phosphate ester bonds to release more inorganic phosphate, which in turn provides the raw material for the formation of eggshell. High levels of estradiol can promote shell gland secretion and deposition, with more calcium and phosphorus, leading to increased toughness and elasticity of the eggshell [26].
The yolk color of eggs from hens fed 1.6% CLA was significantly deepened, which might be attributed to the lipids and antioxidants in the feed, because they can improve the absorption of carotenoids and promote its deposition in the egg yolk. A study showed that CLA is a strong antioxidant [21] and may prevent carotenoids from being oxidized. Therefore, dietary supplementation with CLA can deepen the color of egg yolk.
CLA was found to affect significantly the taste and aroma of egg yolk. On one hand, it due to the change of other chemicals in egg yolk, it can be reduced from compounds such as acetaldehyde, acetone, pentane, acetic acid methyl ester, ethyl acetate, acetone, ethyl acetate, and octane [18,19]. On the other hand, the flavor and taste of CLA are immerged into egg yolk. The combined effect of the two factors perhaps reduces the flavor of egg yolk.
Content and composition of fatty acids in egg yolk
The present study found that, with increasing dietary CLA, SFAs, and PUFAs in egg yolk increased gradually, and unsaturated fatty acids decreased. Ahn [16] also reached the same conclusion. This shows that dietary CLA does have the physiological effect of changing the proportion of SFAs and MUFAs in egg yolk. This may be because delta-9-desaturase, a key enzyme that converts SFAs to the corresponding MUFA, is inhibited by CLA, resulting in increased SFAs and decreased MUFAs. The significant increase in PUFA in egg yolk is probably due to the inhibition of delta-6-desaturase by CLA. Belury and Kempa-Steczko [27] found that CLA is an inhibitor of delta-6-desaturase, a key enzyme that converts linolenic acid into fatty acids of C20:5 series. When delta-6 desaturase is inhibited, hens are not able to convert C18:3 fatty acids to C20:5 fatty acids, leading to increased PUFAs in egg yolk.
Decreased linoleic acid and linolenic acid contents because of dietary CLA supplementation have been reported earlier in poultry [16], and is consistent with our conclusions of the present study. It may be attributed to lower dietary intake of linoleic acid and linolenic acid.
Arachidonic acid in egg yolk decreased after feeding with dietary CLA. This is consistent with the previous results [19,20]. Two mechanisms have been proposed. One is that delta-6 desaturase, as a rate-limiting enzyme in the conversion of linoleic acid and linolenic acid to arachidonic acid, is bound competitively by CLA. The other is that linoleic and linolenic acid are higher in CLA than soybean oil, which substituted for CLA to maintain the calories supplied by each dietary regimen. As precursors of arachidonic acid, lower amounts of CLA therefore meant correspondingly lower amounts of arachidonic acid in egg yolk.
In the present study, the proportions of the CLA isomers cis-9, trans-11, and trans-10, cis-12 in the dietary supplement were very similar (39.7% and 41.21%, respectively), but in egg yolk cis-9, trans-11 accounted for 66% to 70% of the total CLA, and trans-10, cis-12 isomers was 29% to 34%. This suggests that these CLA isomers were differentially deposited. Raes [17] reported that the deposition efficiency of cis-9, trans-10 was higher than that of trans-11, cis-12, which is consistent with our results. However, the reasons for this selectivity remain unclear.
The overall results of this study confirm previous reports [8,9,17,18,22] that increasing CLA in the diet of laying hens increases the concentrations of CLA, SFAs, and PUFAs, and decreases the concentrations of MUFAs in egg yolk. Dietary CLA was found to enhance significantly the eggshell toughness. The incorporation of CLA isomers into egg yolk lipids implies that CLA supplementation could add to the value of chicken eggs for human consumption. However, the adverse effects of dietary CLA on egg quality (such as the odor, flavor, taste) need to be prevented.
ACKNOWLEDGMENTS
This work was supported by a grant (ZR2015YL062) of Natural Foundation of Shandong Province.
Notes
CONFLICT OF INTEREST
We certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.