Lignosulfonic acid promotes hypertrophy in 3T3-L1 cells without increasing lipid content and increases their 2-deoxyglucose uptake
Article information
Abstract
Objective
Adipose tissue plays a key role in the development of obesity and diabetes. We previously reported that lignosulfonic acid suppresses the rise in blood glucose levels through the inhibition of α-glucosidase activity and intestinal glucose absorption. The purpose of this study is to examine further biological activities of lignosulfonic acid.
Methods
In this study, we examined the effect of lignosulfonic acid on differentiation of 3T3-L1 cells.
Results
While lignosulfonic acid inhibited proliferation (mitotic clonal expansion) after induction of differentiation, lignosulfonic acid significantly increased the size of accumulated lipid droplets in the cells. Semi-quantitative reverse transcription polymerase chain reaction analysis showed that lignosulfonic acid increased the expression of the adipogenic transcription factor, peroxisome proliferator-activated receptor gamma (PPARγ), leading to increased glucose transporter 4 (Glut-4) expression and 2-deoxyglucose uptake in differentiated 3T3-L1 cells. Additionally, feeding lignosulfonic acid to diabetic KK-Ay mice suppressed increase of blood glucose level.
Conclusion
Lignosulfonic acid may be useful as a functional anti-diabetic component of food.
INTRODUCTION
Lignins are natural polymers derived from wood. They consist of 4-hydroxyphenyl, guaiacyl, and syringyl structures. Lignins are non-toxic compounds present in a large variety of foods and cereal brands. They are the major components of insoluble dietary fiber resistant to enzymatic digestion. There have been many studies evaluating the efficacy of dietary fiber in the prevention of many diseases, including colon cancer, heart disease, obesity, and diabetes [1]. Although studies about effects of lignins are scarce, some reports suggest that lignin or lignin-carbohydrate complexes may have anti-tumor, anti-microbial, and anti-HIV activities [2–7].
Lignosulfonic acid is a lignin derivative derived from the sulfite pulping of softwood that is used in paper industry. Although lignosulfonic acid as a waste byproduct has been widely used as a plasticizer of concrete [8], as a feed for livestock, and as a raw material in the production of artificial vanilla flavor, vanillin [9], further utilization is desirable.
Adipose tissue plays an important role in the regulation of glucose metabolism and insulin action. In mature adipocytes, glucose transporter type 4 (Glut-4) is highly expressed and responds to insulin to lower blood glucose [10]. Additionally, adipose tissue expresses and secretes adiponectin, which exerts an insulin sensitizing effect [11]. Therefore, adipose tissue dysfunction in obesity and lipodystrophy are closely related to incidence of type 2 diabetes [12,13]. Agonists of peroxisome proliferator-activated receptor gamma (PPARγ) for treating type 2 diabetes such as thiazolidinediones stimulate the differentiation of adipocytes, increasing Glut-4 expression and insulin sensitivity, although they cause a side effect of weight gain [10,14]. Compounds that stimulate preadipocyte differentiation and/or regulate PPARγ target genes are effective as anti-diabetes agents.
After the differentiation of 3T3-L1 preadipocytes was induced with a medium containing insulin, the cells undergo several rounds of mitosis referred to as mitotic clonal expansion at an early event of differentiation [15,16]. A transcription factor, CCAAT/enhancer binding protein (C/EBP)-β, is required for the mitotic clonal expansion [15]. After mitotic clonal expansion, the preadipocyte cells express the key transcription factor, PPARγ, for adipocyte differentiation, which plays essential roles for inducing the adipocyte specific PPAR-γ target genes such as Glut-4 and fatty acid binding protein [10]. The 3T3-L1 cells gradually increase lipid droplet size and number during the differentiation process.
We previously reported that lignosulfonic acid suppresses the rise in blood glucose levels through the inhibition of α-glucosidase activity and intestinal glucose absorption [17]. In this study, we investigated the effect of lignosulfonic acid on the differentiation of 3T3-L1 preadipocytes affecting Glut-4 expression and glucose uptake.
MATERIALS AND METHODS
Materials
Lignosulfonic acid was purchased from Sigma (St. Louis MO, USA). An antibody against β-actin was purchased from Sigma and an antibody against C/EBP-β was purchased from Cell Signaling Technology (Danvers MA, USA). 3T3-L1 preadipocyte cells (JCRB9014) were purchased from the Human Science Research Resource Bank (Osaka, Japan). KK-Ay mice were purchased from CLEA (Tokyo, Japan)
Cell culture
3T3-L1 preadipocytes were cultured as described previously [18]. Briefly, 3T3-L1 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1 mM ascorbic acid at 37°C. To induce adipocyte differentiation, the 3T3-L1 cells were seeded in 24-well plates and then stimulated with 0.25 μM dexamethasone, 0.5 mM 3-isobutyl-1-methylxanthanine, and 1 μM insulin added to DMEM with 10% FBS, in the absence or presence of lignosulfonic acid at the indicated concentrations in Figures. After two days, the medium was replaced with DMEM containing 10% FBS and 1 μM insulin with or without lignosulfonic acid. Cultures were maintained for 7 to 10 days before being used for experiments.
Oil red O staining
Intracellular lipid accumulation was determined by oil red O staining during adipocyte differentiation as described previously [18]. Differentiated 3T3-L1 cells were washed twice with phosphate-buffered saline (PBS) and fixed with a 3.7% formaldehyde solution for 10 min, then stained with a 0.2% filtered oil red O solution. Several fields of the cells were photographed using phase contrast microscopy and then the cell diameter and number were estimated. The cell-incorporated dye was extracted with 60% isopropanol and the absorbance of the extract measured at 490 nm.
Cell viability
Proliferation of 3T3-L1 cells was determined using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay [19]. After the 3T3-L1 cells were differentiated in the absence or presence of lignosulfonic acid at concentrations of 1.0 mg/mL and 2.0 mg/mL for 2 days, MTT was added to each well (final concentration of 0.5 mg/mL) and incubated at 37°C for 4 h. The medium was carefully removed, and the colored product was dissolved in 20% sodium dodecyl sulfate (SDS). After 24 h, the absorbance was measured at 570 nm.
Cytotoxicity
Cytotoxicity was measured by lactate dehydrogenase (LDH) activity. Lignosulfonic acid of 2.0 mg/mL was added to the 3T3-L1 preadipocyte cells after induction of differentiation. After 2 days, LDH activity was measured using an LDH Cytotoxicity Test Kit (Wako, Osaka, Japan).
Measurement of 2-deoxyglucose uptake
The 2-deoxyglucose uptake assay was performed as described previously [17]. Briefly, the cells were seeded at 6×104 cells per well in a 6-well culture plate. 3T3-L1 preadipocytes were then differentiated in the absence or presence of lignosulfonic acid at concentrations of 0.5 mg/mL and 1.0 mg/mL for 8 days. The cells were washed thrice with Krebs-Ringer phosphate buffer (KRP buffer containing 136 mM NaCl, 5 mM Na2HPO4, 4.7 mM KCl, 1 mM MgSO4, 20 mM HEPES-NaOH (pH 7.0) and 1 mM CaCl2), and then incubated in KRP buffer containing 2% bovine serum albumin for 20 min at 37°C. 1 mM of 2-deoxyglucose were added and the cells were incubated for 20 min at 37°C. After removing the solution, cells were washed thrice with PBS and then resuspended in 10 mM Tris-HCl (pH 7.5). After sonication and heat treatment for 15 min at 95°C, homogenized cells were pelleted by centrifugation at 14,000×g for 15 min and the supernatant was recovered. The amount of 2-deoxyglucose in the supernatant was measured using a proprietary 2-deoxyglucose uptake measurement kit (Cosmo bio, Tokyo, Japan).
Western blotting
After 3T3-L1 preadipocyte cells were differentiated in the absence or presence of lignosulfonic acid at concentrations of 0.2 mg/mL and 2.0 mg/mL for 2 days, cells were homogenized in a solution containing 2% SDS, 20 mM Tris-HCl (pH 7.5), 1 mM 2-mercaptoethanol, 10% glycerol, and bromophenol blue, and the cell extracts were subjected to SDS-polyacrylamide gel electrophoresis [20]. Proteins in the gel were transferred to a polyvinylidene difluoride membrane, which was then blocked in 5% skim milk (w/v) in Tris-buffered saline containing 0.5 M NaCl, 20 mM Tris HCl (pH 7.5), and 0.05% Tween 20 (solution A). After incubation of the membrane with β-actin or C/EBP-β antibodies, the alkaline phosphatase-conjugated goat anti-rabbit IgG secondary antibody was added. The color was developed by adding nitroblue tetrazolium and 5-bromo-4-chloro-3-indoyl phosphate. Protein band intensities were measured with Image J (NIH).
Semi-quantitative RT-PCR analysis
After 3T3-L1 preadipocyte cells were differentiated in the absence or presence of lignosulfonic acid at concentrations of 0.2 mg/mL and 2.0 mg/mL, total RNA was purified using an RNAiso Plus (Takara, Shiga, Japan) according to the manufacturer’s protocol. After first-strand cDNA synthesis from 20 μg of total RNA using oligo (dT) primer, polymerase chain reaction (PCR) was carried out using 0.05 μg of cDNA template and specific sense and antisense primers of actin, PPARγ, sterol regulatory element-binding protein (SREBP-1c), and Glut-4 in a final volume of 25 μL. The primer sequences were as follows: forward, 5′-ATGGGTCAGAAGGACTCCTACG -3′, and reverse, 5′-AGTGGTACGACCAGAGGCATAC -3′ for actin; forward, 5′-TTTTCAAGGGTGCAAGTTTCAATCC -3′, and reverse, 5′-AATCCTTGGCCCTCTGAGAT -3′ for PPARγ: forward, 5′-GCTTCTGTTGCCCTTCTGTC -3′, and reverse, 5′-TGGACGCTCTCTTTCCAACT -3′ for Glut-4; forward, 5′-TGTTGGCATCCTGCTATCTG -3′, and reverse, 5′-AGGGAAAGCTTTGGGGTCTA -3′ for SREBP-1c. The PCR cycling conditions were as follows: For actin, 30 cycles; 94°C for 30 s, 58°C for 30 s, 72°C for 30 s; For PPARγ, Glut-4, and SREBP-1c, 30 cycles; 94°C for 30 s, 56°C for 30 s, 72°C for 30 s. The intensities of amplified bands were estimated using Image J software. The Glut-4 and PPARγ mRNA expression levels were normalized to actin mRNA level. The amplification cycles were determined based on the relationship between the amount of PCR product detected and cycle number.
Animals and diet
Four-week-old male KK-Ay mice were housed individually and allowed to acclimate for a week post-arrival in a room maintained at 22°C. The animals were provided with water itum. The mice were cared for in accordance with the Guidelines for Experimental Animal Care issued by the Office of the Prime Minister of Japan. After the acclimatization period, the mice were randomly divided into two groups each of the 5 animals, and fed for 4 weeks with either basal diet (control diet) or diet supplemented with 2% lignosulfonic acid (lignosulfonic acid diet). The basal diet consisted of (in wt.%) casein, 20; cornstarch, 15; corn oil, 5; AIN-76 mineral mixture, 5; AIN-76A vitamin mixture, 1.0; L-cysteine, 0.3; choline, 0.2; sucrose, 47; and cellulose, 3. For the lignosulfonic acid diet or control diet, 2% (wt.) of lignosulfonic acid or cellulose was added to the basal diet, respectively. The mice had free access to food and water. Body weights were measured every week. Blood samples were taken from the tail vein every week. Blood glucose concentration was measured using a glucometer and test-strips (Abbott, IL, USA). The average of three measurements at each time point for each rat was calculated and used for statistical analysis.
Statistical analysis
Each experiment was performed two or thrice. Experiments were performed in duplicate or triplicate. Data were combined from at least four data and expressed as the mean and the standard deviation. Data were analyzed by Student’s t-test.
RESULTS
Effect of lignosulfonic acid on the differentiation of 3T3-L1 preadipocytes
Differentiation of 3T3-L1 preadipocyte cells was induced with insulin, dexamethasone, and 1-methyl-3-isobutylxanthine in the absence or presence of lignosulfonic acid for 6 to 8 days. The 3T3-L1 cells accumulated lipid during the differentiation process. The extent of adipocyte differentiation was evaluated by quantifying the accumulated lipid droplets in 3T3-L1 cells after they have been stained with oil red-O dye. Lignosulfonic acid had no significant effects on the amount of lipid accumulation in 3T3-L1 cells (Figure 1a). However, the size of lipid droplets accumulated in the cells increased to about 3-fold and the number of lipid droplets significantly decreased (Figure 1b and c).
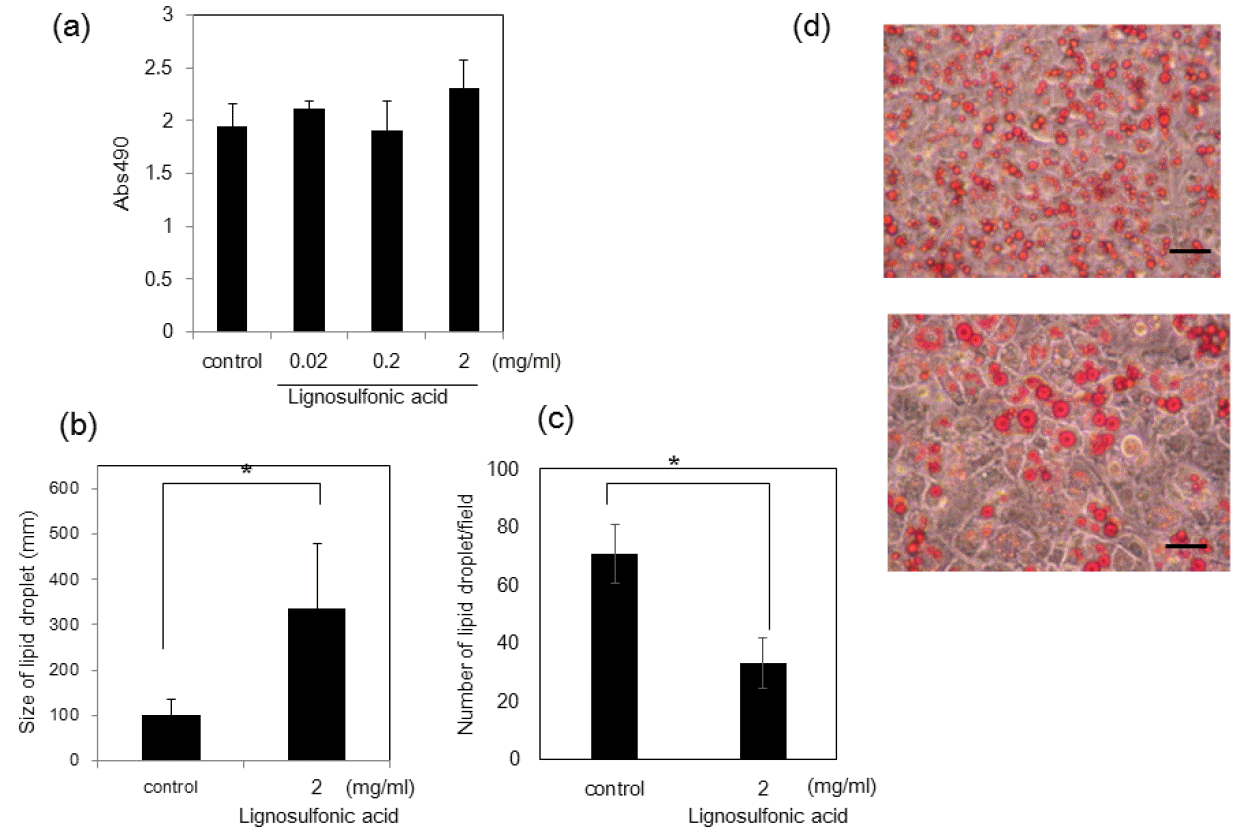
Effect of lignosulfonic acid on the differentiation of 3T3-L1 preadipocytes. (a) 3T3-L1 preadipocyte cells were differentiated in the absence (control) or presence of lignosulfonic acid at the indicated concentrations. The amount of lipid accumulation was estimated using oil red O staining. Data represents an average of measurements taken from 6 wells out of a 24-well plate±standard deviation (SD). Size (b) and the number (c) of lipid droplets in each field were measured. Data were combined from 6 fields and the bars show SD. Statistical significance was evaluated by Student’s t-test (* p<0.05). (d) Phase contrast images of differentiated cells in the absence (upper panel) or the presence (lower panel) of 2 mg/mL lignosulfonic acid. Scale bar represents 25 μm.
Effect of lignosulfonic acid on mitotic clonal expansion
When 3T3-L1 preadipocyte cells are induced to differentiate, the cells are known to undergo several rounds of mitosis referred to as mitotic clonal expansion. We decided to investigate whether the observed decrease in lipid droplet number is due to inhibition of mitotic clonal expansion. Lignosulfonic acid inhibited mitotic clonal expansion to about 60% of control levels at a concentration of 2.0 mg/mL (Figure 2a). In contrast, LDH activity did not significantly increase at the concentration of 2.0 mg/mL that inhibits mitotic clonal expansion (Figure 2b), showing that the decrease of cell number is not due to cell toxicity of lignosulfonic acid. We investigated the expression of CCAAT/enhancer-binding protein β (C/EBP-β), which is required for mitotic clonal expansion [15]. Lignosulfonic acid suppressed the expression level of C/EBP-β to about 25% of the control at a concentration of 2.0 mg/mL (Figure 2c). These results suggest that lignosulfonic acid decreases cell number by the inhibition of mitotic clonal expansion, resulting in the decrease of the number of lipid droplets.
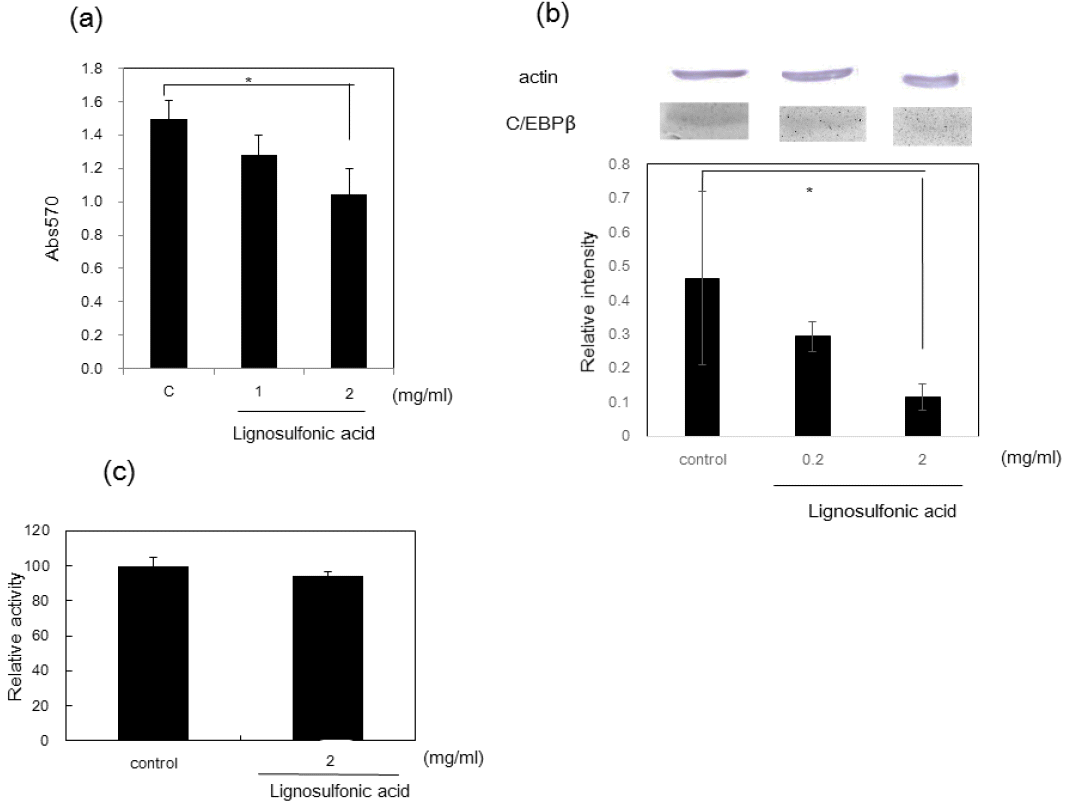
Effect of lignosulfonic acid on preadipocyte proliferation after induction of differentiation. (a) Lignosulfonic acid was added to 3T3-L1 preadipocyte cells at concentrations of 1.0 mg/mL and 2.0 mg/mL. Control shows the data in the absence of lignosulfonic acid. After the cells were maintained for 48 h, proliferation was measured by MTT assay. (b) Effect of lignosulfonic acid on the expression of C/EBP-β. The expression levels of C/EBPβ were examined 48 h after induction of differentiation. (c) LDH content released into culture medium was measured using a LDH cytotoxic test kit 48 h after induction of differentiation. Data were expressed as the mean±standard deviation. Statistical significance was evaluated by Student’s t-test (* p<0.05).
Effect of lignosulfonic acid on the expression levels of the adipogenic transcription factors and glut-4
Next, we investigated whether the increase of size of lipid droplets is due to the promotion of differentiation of 3T3-L1 preadipocytes after mitotic clonal expansion. We examined the expression levels of two adipogenic transcription factors: SREBP-1c and PPAR-γ, which play essential roles in activating the adipocyte specific genes. Semi-quantitative reverse transcription-PCR (RT-PCR) analysis showed that treatment of 3T3-L1 cells with lignosulfonic acid increased the expression of PPARγ and SREBP-1c (Figure 3). Additionally, lignosulfonic acid induced the expression of Glut-4, a target gene of PPARγ, which is tightly associated with glucose transport in adipocytes. To confirm the increase in Glut-4 expression, we investigated 2-deoxyglucose uptake in 3T3-L1 adipocytes. 3T3-L1 adipocytes differentiated in the presence of lignosulfonic acid significantly increased glucose uptake by approximately 2-fold at a concentration of 1.0 mg/mL (Figure 4). These results suggest that lignosulfonic acid promotes the differentiation of 3T3-L1 preadipocytes after mitotic clonal expansion, leading to increased lipid droplet size. Additionally, lignosulfonic acid increases glucose uptake into adipocytes by increasing Glut-4 expression.
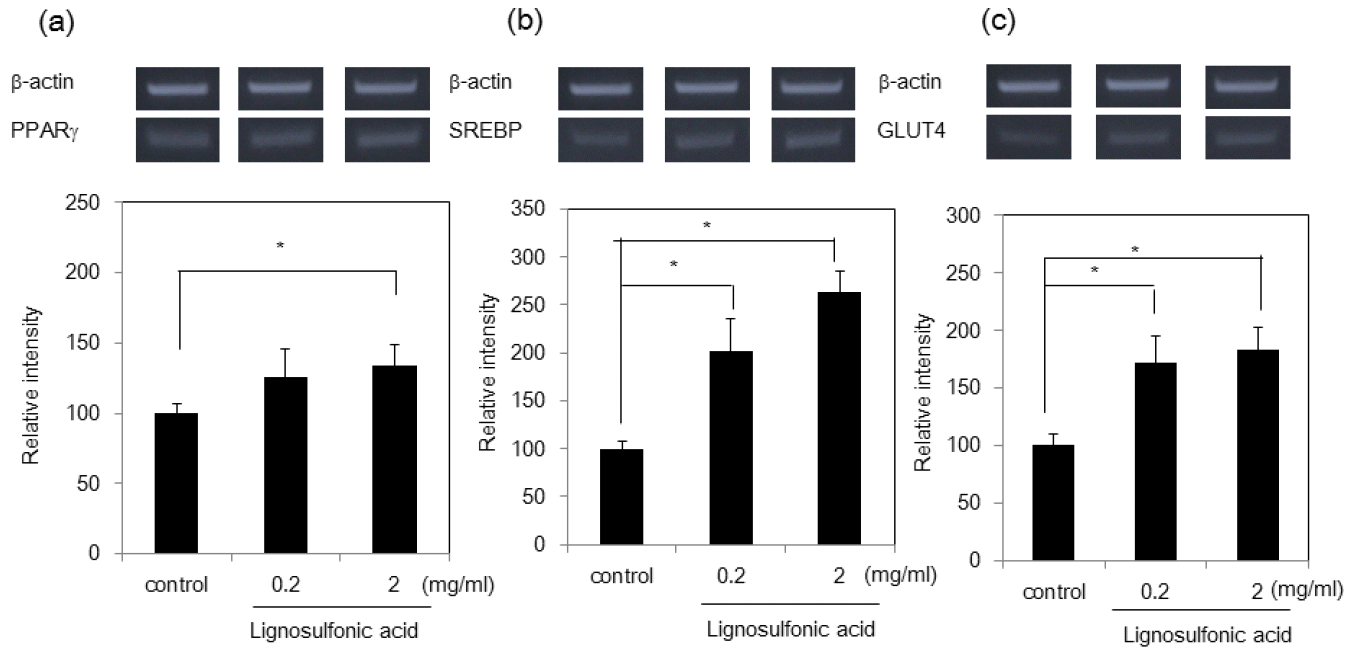
Effect of lignosulfonic acid on the expression of PPARγ (a), SREBP-1c (b), and Glut-4 (c). The 3T3-L1 preadipocyte cells were differentiated in the absence (lane 1) or presence of lignosulfonic acid at the indicated concentrations (lane 3). After 8 days, the mRNA expression levels of PPARγ, SREBP-1c, and Glut-4 were examined by semi-quantitative reverse transcription polymerase chain reaction (RT-PCR). Data were expressed as the mean±standard deviation. Statistical significance was evaluated by Student’s t-test (* p<0.05).
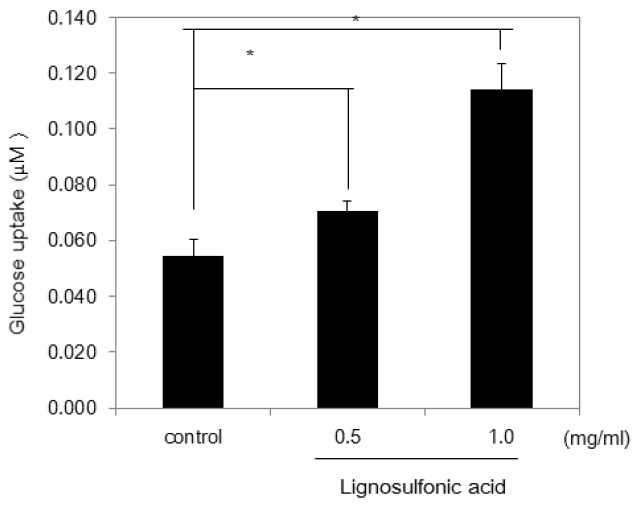
Effect of lignosulfonic acid on 2-deoxyglucose uptake. The amount of 2-deoxyglucose uptake of adipocytes differentiated in the absence or presence of lignosulfonic acid at the indicated concentrations was indicated. Data were expressed as the mean±standard deviation. Statistical significance was evaluated by Student’s t-test (* p<0.05).
Effect of feeding lignosulfonic acid on serum glucose levels in KK-Ay diabetic mice
Since lignosulfonic acid increased glucose uptake in 3T3-L1 preadipocytes, we investigated whether feeding of lignosulfonic acid to KK-Ay diabetic mice would suppress an increase of serum glucose. Mice were fed either a control diet or a diet supplemented with 2% lignosulfonic acid for 8 weeks. The serum glucose levels of KK-Ay mice increased gradually with age (Figure 5a). Lignosulfonic acid significantly suppressed the increase of the serum glucose levels in KK-Ay diabetic mice compared to that of the control. After 8 weeks, blood glucose level of control group was approximately 2-fold greater than that of the lignosulfonic-fed group. However, neither body weight nor weight of adipose tissue-to-body weight ratios differed significantly between the lignosulfonic acid and control groups after 8 weeks (Figure 5b and c).
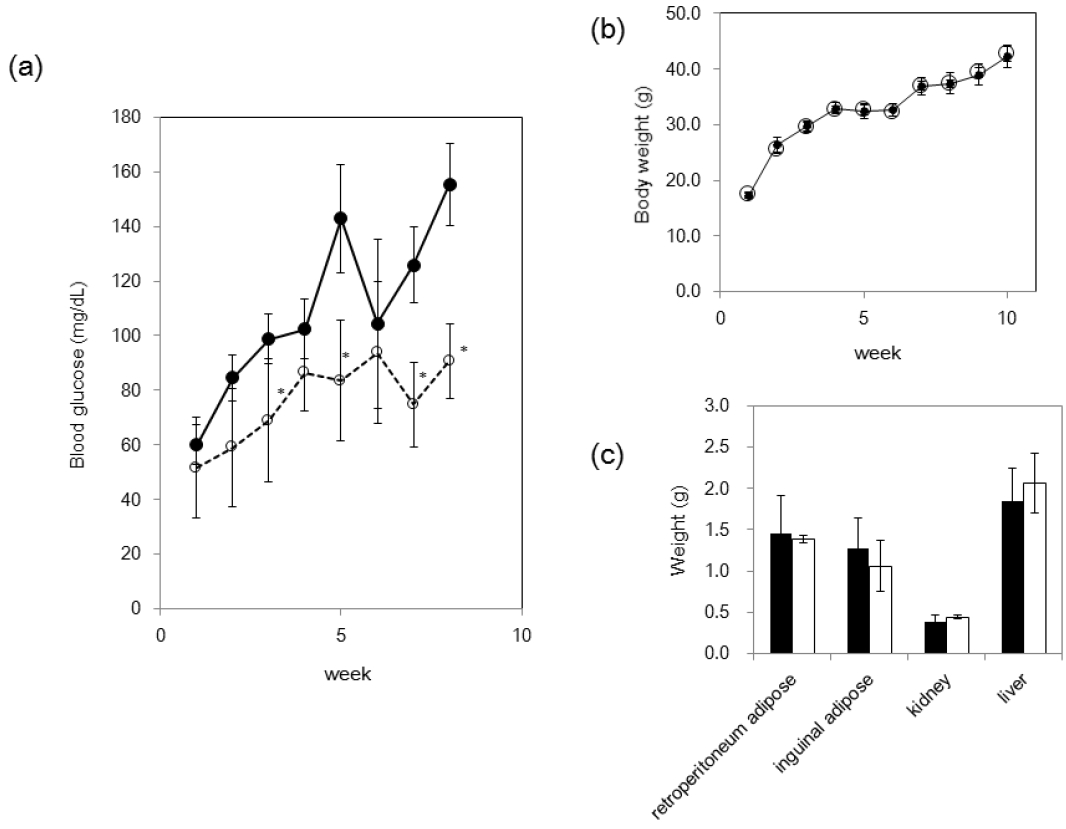
Effect of feeding of lignosulfonic acid in KK-Ay mice. (a) Blood glucose level in KK-Ay mice fed the control diet (closed circle) or the lignosulfonic acid diet (open circle). (b) Body weight of mice fed with lignosulfonic acid diet (open circle) or control diet (closed circle) was measured weekly. (c) Weights of kidney, liver, and adipose tissues were measured 8 weeks after initiation of feeding of lignosulfonic acid diet (open bar) or control diet (closed bar). Data were expressed as the mean±standard deviation. Statistical significance was evaluated by Student’s t-test (* p<0.05).
DISCUSSION
Treatment with compounds inducing differentiation of 3T3-L1 preadipocytes leads to mitotic clonal expansion prior to the expression of PPARγ, a key adipogenic transcription factor. Many compounds or extracts have been reported to regulate the differentiation of 3T3-L1 preadipocytes by affecting mitotic clonal expansion. Some compounds inhibited the differentiation of 3T3-L1 preadipocytes by suppressing the mitotic clonal expansion [21–24]. The orphan nuclear receptor Nur77 accelerated the differentiation by promoting mitotic clonal expansion [25]. On the other hand, PD98059 and U0126 (an inhibitor of mitogen-activated protein kinase (MEK)/extracellular signal-regulated kinase [Erk] 1) inhibited mitotic clonal expansion, but did not affect the preadipocyte differentiation [16,26]. Lignosulfonic acid inhibited mitotic clonal expansion, but promoted preadipocyte differentiation. These studies suggest that the inhibition of mitotic clonal expansion does not always lead to the inhibition of preadipocyte differentiation. C/EBPβ, which is expressed immediately after induction of differentiation, acts as a transcriptional activator of PPARγ. However, the expression of PPARγ is not regulated by C/EBPβ alone. A small molecule harmine induced the expression of PPAR without affecting the expression of C/EBPβ through Wnt signaling pathway [27]. PD98059 suppressed the expression of PPARγ without affecting the expression of C/EBPβ through the MEK/Erk signaling pathway. In this study, lignosulfonic acid reduced the expression of C/EBPβ, but increased the PPARγ expression. Lignosulfonic acid may affect, not only the C/EBPβ expression process, but also the MEK/Erk signaling pathway or Wnt signaling pathway. Further investigation will be required to clarify the action mechanism of lignosulfonic acid.
Our data also show that feeding lignosulfonic acid to KK-Ay diabetic mice suppressed the increase of serum glucose level seen in control animals. The average molecular weight of the lignosulfonic acid used is about 8,000 Da, which is likely to be too large for absorption via the small intestine. Since lignosulfonic acid had a wide range of molecular weights, lignosulfonic acid molecules with low molecular weight may be absorbed and affect adipose tissue. We reported previously that lignosulfonic acid suppresses the rise in blood glucose levels after oral administration of sucrose or glucose through inhibition of α-glucosidase activity and intestinal glucose absorption [17]. Suppression of the increase of serum glucose level in KK-Ay mice by lignosulfonic acid may be caused by the inhibition of α-glucosidase activity and intestinal glucose absorption.
While PPARγ agonists for treating type 2 diabetes increase glucose uptake and insulin sensitivity by stimulating adipogenesis, they increase lipid accumulation in adipocytes and cause weight gain [10,14]. In contrast, lignosulfonic acid increases PPARγ expression during the differentiation of 3T3-L1 preadipocytes, but does not increase the amount of accumulated lipid. If lignosulfonic acid can act on adipose tissue in vivo, it may be useful as an anti-diabetes agent, having several functions including inhibiting α-glucosidase activity and intestinal glucose absorption, and promoting glucose uptake in adipocytes without causing a weight gain. Attempts to clarify the action for adipose tissue of lignosulfonic acid in vivo are ongoing.
CONCLUSION
While lignosulfonic acid treatment inhibited proliferation (mitotic clonal expansion) after induction of differentiation, it significantly increased the expressions of PPARγ and Glut-4, and glucose uptake into adipocytes. Additionally, feeding lignosulfonic acid to KK-Ay diabetic mice significantly suppressed the increase of the serum glucose levels observed in control animals. These results suggest that lignosulfonic acid is effective as a functional food supplement preventing the increase of serum glucose.
Notes
CONFLICT OF INTEREST
We certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.