Effects of Short-term Feeding Magnesium before Slaughter on Blood Metabolites and Postmortem Muscle Traits of Halothane-carrier Pigs
Article information
Abstract
Fifty-four, mixed-sex, halothane-carrier crossbred (Yorkshire×Landrace) pigs with an average initial BW of 108.2±0.8 kg were randomly allotted to one of three dietary treatments for 5 d before slaughter: i) a control corn-soybean meal finisher diet devoid of supplemental magnesium; ii) a diet supplemented with 1.5 g/kg of elemental Mg from magnesium acetate; and iii) a diet supplemented with 1.5 g/kg of elemental Mg from magnesium sulfate heptahydrate. Serum creatine kinase (CK), lactate and glucose were analyzed at slaughter. Muscles from longissimus (LM) were packaged and stored to simulate display storage for muscle lactate and glycogen determinations at 0, 1, 2, 3, and 4 d. Mg supplementation reduced (p<0.05) serum CK and lactate concentration, but had no effect (p>0.05) on serum glucose. Daily change of muscle lactate concentration linearly increased (p<0.01), while glucose concentration linearly decreased (p<0.05) as storage time increased in all treatments. However, dietary Mg acetate and Mg sulfate supplementation in pigs elevated (p<0.05) muscle glycogen and reduced (p<0.05) muscle lactate concentrations, especially during the first 2 d of display, compared with pigs fed the control diet. This study suggests that short-term feeding of magnesium acetate and magnesium sulfate to heterozygous carriers of the halothane gene has beneficial effects on stress response and pork quality by improving blood and muscle biochemical indexes.
INTRODUCTION
The pre-slaughter handling of fattening pigs is one of the most stressful events and it has an important effect on the final carcass and pork quality (Gispert et al., 2000; Panella-Riera et al., 2008). It includes mixing unfamiliar animals, fasting, loading, transport, abattoir lairage and the stunning procedure. All of these are stressful factors which sometimes are difficult to alleviate. Acute pre-slaughter stress may result in an increase in drip loss and paler meat (Panella-Riera et al., 2008), producing pale, soft and exudative (PSE) meat. The existence of PSE meat also has a genetic origin related to the halothane gene (n) which is also known as RYR1 gene. In fact, homozygous halothane positive (nn) and heterozygous (Nn) pigs have an abnormality in their muscle metabolism that makes them particularly sensitive to acutely stressful stimuli, causing a higher incidence of PSE meat (Jensen and Barton-Gade, 1985; Oliver et al., 1993; Fàbrega et al., 2002). This abnormality does not exist in halothane negative (NN) pigs. Fàbrega et al. (2004) observed that Nn pigs had higher heart rate and CPK than NN pigs, which indicated that halothane carriers may be more responsive to stressful situations and this could compromise the welfare of those individuals.
Research has confirmed that stress associated effects on animal blood and muscle biochemical traits may be improved by including feed components such as Mg in the diet of pigs, especially those with the halothane gene. Magnesium (Mg) is reported to be an important cofactor in many enzymatic reactions involved in energy and protein metabolism and can counteract catecholamine effects in stress situations (Kietzmann and Jablonski, 1985; Sahin et al., 2005). In fact, short-term supplemental Mg has been reported to alleviate the acute stress response resulting from handling prior to slaughter (Kuhn et al., 1981; Peeters et al., 2005) and improve pork quality (Caine et al., 2000; Hamilton et al., 2002; Frederick et al., 2006; Alonso et al., 2012). However, different results have been obtained when comparing the effect of Mg supplementation to carriers (Nn) of the halothane gene (Caine et al., 2000; Apple et al., 2002), which may be due to the difference of Mg sources (organic Mg or inorganic Mg).
Therefore, the aim of this study was to assess the effect of short-term supplementation of swine finishing diets with different Mg sources (magnesium acetate and magnesium sulfate) on serum biochemical traits and muscle post mortem changes of heterozygous pigs of the Halothane gene under generally commercial conditions.
MATERIALS AND METHODS
Halothane genotype determination
The halothane genotype was determined by using a biopsy sample of blood obtained through an ear vein puncture by DNA tests (Blood Genome DNA Extraction Kit and TaKaRa PCR Amplification Kit, TaKaRa Biotechnology (Dalian) Co., Ltd.) according to Fujii et al. (1991) when the pigs were approximately 40 kg live weight. Pigs selected in experiment were all heterozygous pigs (halothane-carrier pigs).
Animals and treatments
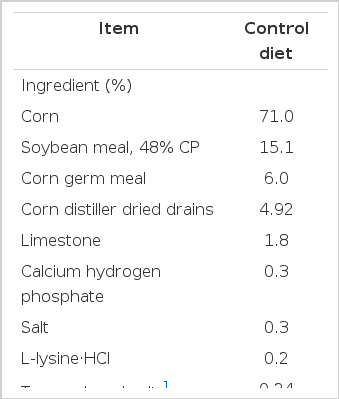
Fifty-four, mixed-sex, halothane-carrier crossbred (Yorkshire×Landrace) pigs with an average initial BW of 108.2±0.8 kg were randomly allotted to one of three dietary treatments for 5 d before slaughter: i) a control corn-soybean meal finisher diet devoid of supplemental magnesium; ii) a diet supplemented with 1.5 g/kg of elemental Mg from magnesium acetate (MgAc, 11.2% Mg); and iii) a diet supplemented with 1.5 g/kg of elemental Mg from magnesium sulfate heptahydrate (MgSO4·7H2O, 9.6% Mg). Each treatment was replicated six pens with three pigs per pen.
Housing and feeding
Animals were housed in buildings on partially slatted concrete floors, and each pen was 1.80×3.00 m with a floor space allowance of 1.8 m2/ pig. The environment within the building was controlled using a thermostat and fan ventilation (the building temperature remained between 19°C and 20°C, and the relative humidity remained between 50% and 55%).
The finisher diet used in the experiment was formulated to meet or exceed NRC (1998) requirements for growing-finishing swine. Feed in the form of a standard pelleted finisher diet (Control diet; Table 1) was offered ad libitum and water was freely available from a nipple drinker.
Slaughter, fabrication and storage
Pigs were transported (15±5 min) to a commercial abattoir over a distance of approximately 7 km and were kept overnight in lairage (time off feed-12 h). Slaughter took place on the same day. Pigs were stunned electrically (240 V, 800 Hz for 2 s) and killed by exsanguinations. At slaughter, blood samples (10 ml) and LM from the left side of each carcass of each pig were collected and transported to the Animal Production Laboratory of Shenyang Agricultural University. Blood for the analysis of serum creatine kinase (CK), glucose, lactate was transferred into a plain glass storage tube and was kept on ice until centrifugation. Tubes were centrifuged at 1,500×g at 4°C for 20 min, and serum was stored at −20°C before analysis. The LM was sectioned into chops for muscle lactate, glycogen. All packages were placed on an absorbent pad within a styrofoam tray, wrapped with an oxygen-permeable film and stored in a refrigerator at 4±1°C in the presence of fluorescent lighting to simulate retail display during 5 d of storage time. Muscle chemical analysis was performed on d 0, 1, 2, 3 and 4 of storage.
Chemical analyses
Serum glucose concentrations were determined by using a spectrophotometric procedure in commercially available kit (Sigma Chemical Co., St. Louis, MO, USA). Serum lactate concentrations were determined spectrophotometrically by using the procedure of Brandt et al. (1980), modified to use microtiter plates, whereas serum CK concentrations were determined using a spectrophotometric procedure from commercially available kits (Sigma Chemical Co., St. Louis, MO, USA).
The muscle lactate concentrations were measured by using a commercially available lactate kit (826-A; Sigma-Aldrich Co.) by the change in spectrophotometric absorbance of NADH at 340 nm (Bergmeyer, 1974). Glycogen concentrations were determined simultaneously by the change in absorbance at 340 nm (Dalrymple and Hamm, 1973).
Statistical analysis
All experimental data were analyzed by the General Linear Model (GLM) procedure of SAS (SAS Inst., Inc., Cary, NC, 1996), with dietary treatment and storage period as the main effect and also their interaction in the model. Differences between treatments were determined by using Duncan’s test. Polynomial contrasts (linear or quadratic) were conducted to evaluate the effect of display time. A level of p<0.05 was set as the criterion for statistical significance. A pen was the experimental unit.
RESULTS
Serum CK, lactate and glucose concentrations
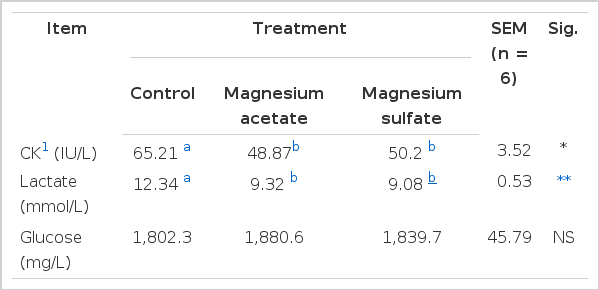
The results of serum CK, glucose, and lactate are presented in Table 2. Pigs fed diets supplemented with Mg acetate and Mg sulfate had lower (p<0.05) serum CK and lactate concentrations compared to pigs fed the control diet. However, there were no differences (p>0.05) in serum glucose concentrations between pigs fed the control diet compared with pigs fed the different Mg diets.
Muscle lactate and glycogen concentrations
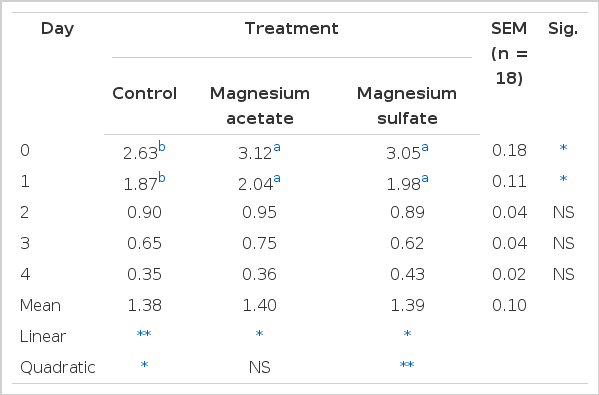
Muscle lactate data are presented in Table 3. Dietary Mg supplementation (p<0.05) and storage period (p<0.05) influenced muscle lactate, but no interaction occurred (p>0.05). Pigs that were fed the Mg acetate and Mg sulfate supplemented diets had lower muscle lactate at 0 (p<0.05) and 1 (p<0.01) d of storage compared with pigs that were fed the control diet. There were no differences (p>0.05) in muscle lactate concentrations between pigs fed the control diet compared with pigs fed the different magnesium diets from 2 to 4 d of storage. Daily change (0 to 4 d of storage) of muscle lactate showed significantly linear increase in the control group (p<0.01), Mg acetate diet treatment (p<0.01), and Mg sulfate diet treatment (p<0.01). There were also significant (p<0.01) quadratic increases in the control group (p<0.01).

The effect of dietary magnesium supplementation on muscle lactate following 0 to 4 d of storage at 4°C (mmol/g)
Muscle glycogen data are presented in Table 4. Dietary Mg supplementation (p<0.05) and storage period (p<0.05) influenced muscle glycogen, but no interaction occurred (p>0.05). Pigs fed the Mg acetate and Mg sulfate supplemented diets had higher (p<0.05) muscle glycogen concentrations compared to pigs fed the control diet on 0 and 1 d of storage. Daily change (0 to 4 d of storage) of muscle glycogen showed significantly linear decrease in the control group (p<0.01), Mg acetate diet treatment (p<0.05) and Mg sulfate diet treatment (p<0.05). There were also significant quadratic decrease in the control (p<0.05) and Mg sulfate diet treatment (p<0.01).
DISCUSSION
Effect of magnesium supplementation on serum metabolites
Stress assessment using blood indices is likely to present some problems when interpreting the results, because stress systems have been said to be affected differently by the same quality of stressor (Schrader and Ladewig, 1999). Creatine kinases (CK) and lactate level are commonly used as indicators of muscle damage (Ryan, et al., 2009) and are released into the blood under certain circumstances such as slaughter stress. Mg is reported to be an important cofactor in many enzymatic reactions involved in energy and protein metabolism and can counteract catecholamine effects in stress situations, and thus decrease the acute stress response resulting from handling prior to slaughter (Panella-Riera et al., 2008). In the present study, the observation that dietary Mg supplementation, whether Mg acetate or Mg sulfate, significantly reduced serum CK and lactate concentrations at slaughter is consistent with Otten et al. (1993, 1995), which reported that dietary Mg fumarate lowered blood lactate concentrations at slaughter during the growing and finishing phase, which indicated that these pigs fed Mg supplementation fought less and were more relaxed. However, Peeters et al. (2006) confirmed that blood CK concentrations did not differ between the Mg acetate supplemented and non-supplemented group through drinking water for Nn pigs. Apple et al. (2005) and Geesink et al. (2004) also reported that Mg mica and Mg acetate did not affect blood lactate concentrations.
There are conflicting results concerning the effect of Mg on the blood glucose concentrations in the pig. In agreement with the present results, Porta et al. (1991) demonstrated that feeding rats high levels (9,000 mg/kg Mg) of supplemental Mg aspartate did not alter blood glucose concentrations. Geesink et al. (2004) and Peeters et al. (2006) reported that there were no differences in blood glucose between the control and Mg-supplemented pigs at slaughter. Haenni et al. (1998) reported no relationship between elevated blood Mg levels and glucose disappearance during a glucose tolerance test. However, Otten et al. (1993, 1995) reported that feeding pigs Mg fumarate from 30 to 100 kg BW significantly lowered plasma glucose concentrations.
Muscle energy metabolism
Muscle glycogen and lactate reflect changes in muscle energy metabolism. Muscle lactate concentration increased as storage time increased, while the opposite trend was detected for glycogen level, which indicated the postmortem energy metabolism of muscles converts glycogen into lactic acid through the glycolytic pathway (Kylä-Puhju et al., 2004). An acute stressor such as negative handling of pigs just prior to slaughter has been shown to increase muscle glycogenolysis (Grandin, 1980). In the present experiment, dietary Mg acetate and Mg sulfate supplementation in pigs elevated muscle glycogen and reduced muscle lactate concentration, especially during the first 2 d of display, compared with pigs fed the control diet, which indicated that Mg delay the initiation of glycolysis by maintaining high energy phosphates postmortem (Moesgaard et al., 1993). The concentrations of glycogen and lactate in muscle were similar between pigs fed the control diet, Mg acetate diet and Mg sulfate diet at the end of storage. This result may indicate that the effect of Mg acetate and Mg sulfate on glycolysis or muscle energy metabolism occurs during relatively short periods of displayed storage (about 2 d after slaughter), and the effect is less noticeable during longer periods of storage.
Because Mg is involved in the activation of several metabolic enzymes, Heaton (1973) concluded that increasing cellular Mg concentrations would result in greater energy utilization and efficiency. In the present study, pigs fed the Mg supplemented diets had less lactate accumulation in muscle suggest less glycolysis occurred and more glycogen reserves compared with pigs fed the control diet. This might be one of the reasons why Mg improved meat quality and reduced PSE meat incidence. Similarly, D’Souza et al. (1999) reported that pigs fed Mg diets (Mg aspartate, Mg sulfate and Mg chloride) had higher muscle glycogen at 5 min and at 40 min (except Mg chloride) post-slaughter and lower muscle lactic acid concentrations in the LT at 5 min post-slaughter compared to pigs fed the control diet. In addition, Lim et al. (2004) also found that supplementing swine diets with Mg sulfate within 1 wk of slaughter resulted in greater glycogen reserves in the LM. However, D’Souza et al. (2000) and Gaine et al. (2000) reported that supplemental dietary Mg actually increased muscle lactate concentrations early postmortem. Apple et al. (2005) reported that the LM from pigs fed 2.5% Mg mica (MM) increased LM lactate concentrations after transportation stress, and tended (p = 0.07) to have higher carbohydrate reserves than those fed 0.0% MM. O’Quinn et al. (2000) failed to detect an effect of supplementary Mg on LM lactate. The conflicting reports on Mg-supplementation may be related to Mg sources, genotype and pre-slaughter handling of pigs.
Magnesium and halothane gene
The scientific literature on the characteristics of heterozygous pigs (Nn) is more controversial. Experiments have confirmed the intermediate position of pigs heterozygous for the HAL gene, as compared with normal and HAL sensitive animals, in terms of post mortem changes in muscle and related quality traits such as meat color, drip loss and sensory-evaluated tenderness (Channon et al., 2000; Fernandez et al., 2002). Studies indicate that halothane carriers (Nn) do have certain advantages compared with halothane negative (NN) pigs such as better feed efficiency, greater carcass yield and higher carcass lean contents (Fisher et al., 2000; Fàbrega et al., 2002). However, these effects were linked to a higher incidence of PSE meat.
The incorporation of supplemental magnesium into animal diets has traditionally been used to prevent the metabolic disorders associated with Mg deficiency (Littledike et al., 1983). However, recent information implied that the magnitude of responses to Mg treatment on pork quality may be related to the stress susceptibility, or resistance, of the pigs in the experiments (Caine et al., 2000; Apple et al., 2002; Panella-Riera et al., 2008; 2009). Campion et al. (1971) reported that the initial and ultimate muscle pH was greater in stress-susceptible pigs intravenously infused with Mg chloride immediately before slaughter, but the muscle pH was unaffected by Mg infusion in stress-resistant pigs. Schmitten et al. (1984) discovered that inclusion of Mg aspartate in swine finishing diets resulted in improvements in pork color and moisture retention in halothane-positive pigs rather than halothane-negative pigs. Moreover, Schaefer et al. (1993) reported that pork color and water-holding capacity had been improved by dietary Mg aspartate only in heterozygous carriers of the halothane gene. Similarly, our data concluded that dietary Mg supplementation improved blood stress indices and muscle energy metabolism of heterozygous carriers of the halothane gene. However, Apple et al. (2002) observed that neither stress response nor postmortem muscle metabolism of halothane-carrier pigs were affected by dietary MM when the pigs were well rested before slaughter.
CONCLUSION
Supplementation with different Mg sources (Mg sulfate and Mg acetate) during the last five days before slaughter improves stress response of halothane-carrier pigs by decreasing serum CK and lactate concentrations. On the other hand, results of this study suggest that supplementation of halothane-carrier pigs diets with Mg sulfate and Mg acetate may be effective in delaying the initiation of glycolysis by elevating muscle glycogen and reducing muscle lactate concentrations. Furthermore, other sources of Mg should be considered for further studies, with the aim of reducing stress before slaughter and being able to reduce the negative effect of the use of the halothane gene.
Acknowledgements
This work was funded by National Key Technology R&D Program in the 12th Five plan of China (Nos.2011BAD28B01-02) and part by Key Program from Liaoning (Nos. 2011202005 and 2011215016) .