Zinc-chelated Vitamin C Stimulates Adipogenesis of 3T3-L1 Cells
Article information
Abstract
Adipose tissue development and function play a critical role in the regulation of energy balance, lipid metabolism, and the pathophysiology of metabolic syndromes. Although the effect of zinc ascorbate supplementation in diabetes or glycemic control is known in humans, the underlying mechanism is not well described. Here, we investigated the effect of a zinc-chelated vitamin C (ZnC) compound on the adipogenic differentiation of 3T3-L1 preadipocytes. Treatment with ZnC for 8 d significantly promoted adipogenesis, which was characterized by increased glycerol-3-phosphate dehydrogenase activity and intracellular lipid accumulation in 3T3-L1 cells. Meanwhile, ZnC induced a pronounced up-regulation of the expression of glucose transporter type 4 (GLUT4) and the adipocyte-specific gene adipocyte protein 2 (aP2). Analysis of mRNA and protein levels further showed that ZnC increased the sequential expression of peroxisome proliferator-activated receptor gamma (PPARγ) and CCAAT/enhancer-binding protein alpha (C/EBPα), the key transcription factors of adipogenesis. These results indicate that ZnC could promote adipogenesis through PPARγ and C/EBPα, which act synergistically for the expression of aP2 and GLUT4, leading to the generation of insulin-responsive adipocytes and can thereby be useful as a novel therapeutic agent for the management of diabetes and related metabolic disorders.
INTRODUCTION
Type 2 diabetes mellitus (T2DM) has become a major pandemic in the last decade with a rate of 3 new cases every 10 seconds (International Diabetes Federation, Diabetes Atlas (5th edition), 2011). Obesity is considered as the most common predecessor of diabetes and cardiovascular disorders; however, lipoatrophy, i.e., the lack of adipose tissue, is also responsible for diabetes and associated metabolic disorders (Reitman et al., 2000). Adipose tissue plays an essential role in energy homeostasis, lipid metabolism, and insulin action as a metabolic and endocrine organ (Kiess et al., 2008). Previous studies have reported that an increase in the number and size of adipose tissue, especially in appropriate adipose beds, could improve its ability to store excess lipids and reduce the deleterious accumulation of triglycerides in muscle, liver, and pancreatic islets (Yamauchi et al., 2001). Accordingly, adipocytes have become a major drug target for diabetes and obesity-related metabolic disorders (Nawrocki and Scherer, 2005). Adipogenesis, the process of converting preadipocytes to mature adipocytes with the consecutive stimulation of adipogenic transcription factors such as CCAAT/enhancer-binding proteins (C/EBPs) and peroxisome proliferator-activated receptor γ (PPARγ), can also lead to the induction of insulin sensitivity (Spiegelman and Flier, 1996). Insulin acts in adipocytes by triggering a cascade of signaling events that are linked with the translocation of insulin-responsive glucose transporter 4 (GLUT4) from intracellular vesicles to the plasma membrane, allowing the uptake of glucose (Holman and Kasuga, 1997). Transcription factors such as CCAAT/enhancer-binding protein alpha (C/EBPα) and PPARγ act synergistically to mediate the regulation of several genes that are responsible for maintenance, creation of the adipocyte phenotype, and glucose and lipid metabolism, e.g., the genes for adipocyte fatty acid binding protein (aP2), GLUT4, lipoprotein lipase, and leptin (Tontonoz et al., 1994). Thiazolidinediones, which are anti-diabetic agents, accelerate adipocyte differentiation, enhance insulin sensitivity, and reduce plasma glucose concentrations by increasing PPARγ expression (Spiegelman, 1998; Willson et al., 2001). GLUT4, which is produced during adipocyte differentiation, is tightly associated with basal and insulin-mediated glucose transport in adipocytes (Shang et al., 2007).
Zinc ascorbate (ZnC) is a chelated compound that is produced by the combination of zinc with vitamin C. ZnC is used as a dietary supplement (antioxidant and immune booster) worldwide. Diabetes patients reportedly have lower serum zinc levels due to increased urinary zinc excretion compared to normal individuals (Kinlaw et al., 1983). An infusion of vitamin C reportedly produces clinical improvement in DM patients (Kodama et al., 1993). A previous study reported that ZnC helped to improve glomerular function in T2DM patients (Farvid et al., 2005). In ZnC, vitamin C is responsible for the enhancement of zinc absorption by cells, while zinc minimizes the fast oxidation of vitamin C. Although the effect of zinc and other multi-vitamin mineral supplements in diabetes or glycemic control is known in humans (Gunasekara et al., 2011; Kodama et al., 1993; May and Contoreggi, 1982; Pittas et al., 2007), the underlying mechanism is not well described. In this study, we investigated the effects of ZnC on adipogenesis and elucidated its molecular mechanism in 3T3-L1 cells.
MATERIALS AND METHODS
Materials
All chemicals and solvents used in the syntheses were of reagent grade and were used without further purification. Elemental analysis was carried out using a LECO CHNS-932 elemental analyzer. Thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) were performed under at a scan rate of 10°C/min using a N2 Scinco TGA 1000 thermal analyzer at Konyang University, Korea.
Preparation of the zinc-chelated vitamin C compound
A solution of ZnSO4 (330 g) in 600 ml water was heated to 50°C and 240 g ascorbic acid were added. The mixture was stirred for an additional 30 min at 70°C. Silica gel (430 g) was then added slowly and the white mixture was dried at 40°C for 24 h.
Thermogravimetric analysis and differential scanning calorimetry
DSC and TGA of ZnC were obtained using a TA Instruments Scinco TGA 1000 thermal analyzer. TGA was performed with a temperature ramp of 10°C/min and a resolution of 6.0°C from room temperature to 1,000°C in a high-purity flowing nitrogen atmosphere (80 cm3/min). Approximately 40 mg of sample were heated in an open platinum crucible.
Cell culture and differentiation
3T3-L1 cells were purchased from the American Type Culture Collection (Manassas, VA, USA). 3T3-L1 mouse fibroblasts were maintained in Dulbecco’s modified Eagle’s medium (DMEM; GIBCO BRL, USA) with 10% bovine calf serum in a humidified atmosphere containing 5% CO2 in air at 37°C. Two days after confluence, adipogenesis was induced using a differentiation mixture containing 10 μg/ml insulin, 0.25 μM dexamethasone, and 0.5 mM 3-isobutyl-1-methylxanthine in DMEM containing 10% fetal bovine serum (FBS). Thereafter, the medium was changed with 10% FBS/DMEM containing 10 μg/ml insulin every 2 d. The test compounds were administered at the initiation of differentiation and with every medium change for 8 d.
Cell viability assay
Cell viability was determined using a Cell Counting Kit (CCK)-8 (Dojindo Laboratories, Japan) according to the manufacturer’s instruction. Briefly, 3T3-L1 cells stimulated by the adipogenic cocktail in the absence or presence of different concentrations of ZnC (0.012 and 0.025 mg/ml) were employed for this experiment in 96-well plates. After each indicated time period, the media containing the test compounds were replaced with media containing 10 μl CCK-8 reagent and incubated in the dark for 2 h. The amount of formazan dye was measured by absorbance at 450 nm using an enzyme-linked immunosorbent assay microplate reader (Infinite F50; Tecan). Each assay was carried out in triplicate.
Glycerol-3-phosphate dehydrogenase activity
Glycerol-3-phosphate dehydrogenase activity (GPDH) activity was measured using a GPDH Activity Assay Kit (TaKaRa, Japan) following the manufacturer’s instructions. One unit is defined as the amount of enzyme required for the consumption of 1 μmol NADH in 1 min at 30°C. Each assay was carried out in triplicate.
Oil Red O staining
Oil Red O staining was performed according to a modified protocol from Mizuarai and associates (Mizuarai et al., 2005). In brief, after differentiation, the cells were washed with phosphate-buffered saline (PBS) and then fixed with 10% formalin in PBS for 60 min at room temperature. The fixed cells were washed 3 times with PBS, stained with a filtered Oil Red O solution for 30 min at room temperature, and rinsed twice with distilled water. Cell morphology was assessed using an Olympus BX51 microscope with imaging software. Finally, the stained cells were destained with isopropanol and the optical density (OD) of the destaining isopropanol was measured using a spectrophotometer at a wavelength of 510 nm.
RNA extraction and cDNA synthesis
Confluent cultures of 3T3-L1 cells in 6-well plates were induced differentiation as described previously. Total RNA was isolated from 3T3-L1 adipocytes using the RNAiso Plus Reagent (Takara, Japan). We reverse transcribed 1 μg RNA using a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, USA) to obtain cDNA according to the manufacturer’s protocols. Briefly, the total reaction volume was 20 μl and the reaction was incubated as follows in a TC-E-96G Gene Pro (Bioer Technology, China): 10 min at 25°C, 120 min at 37°C, 5 min at 85°C, and holding at 4°C.
Real-time PCR (qPCR)
qPCR was performed on an Applied Biosystems 7300 Real-time PCR System (Applied Biosystems, USA) using Power SYBR Green PCR Master Mix (Applied Biosystems, USA) according to the manufacturer’s protocol. Briefly, PCR was performed in a final volume of 20 μl including 10 ng sample cDNA, 5 μM specific forward and reverse primers, and 10 μl Power SYBR Green PCR Master Mix. PCR reactions consisted of an initial denaturing cycle at 95°C for 10 min, followed by 40 amplification cycles: 15 s at 95°C and 1 min at 60°C. The following primers were used. C/EBPα forward: TGG ACA AGA ACA GCA ACG AG; C/EBPα reverse: TCA CTG GTC AAC TCC AGC AC; PPARγ forward: GAT GGA AGA CCA CTC GCA TT; PPARγ reverse: AAC CAT TGG GTC AGC TCT TG; aP2 forward: ATC AGC GTA AAT GGG GAT TTG G; aP2 reverse: GTC TGC GGT GAT TTC ATC GAA; GLUT4 forward: CTT CTT TGA GAT TGG CCC TGG; GLUT4 reverse: AGG TGA AGA TGA AGA AGC CAA GC; β-actin forward: CAC CCC AGC CAT GTA CGT; and β-actin reverse: GTC CAG ACG CAG GAT GGC. The expression levels of C/EBPα, PPARγ, aP2, and GLUT4 were normalized using β-actin as an internal control. Analysis was carried out in triplicates.
Western blot analysis
The cells were seeded in a 6-well plate and adipocyte differentiation was induced as described above. Different concentrations of ZnC (0.012 and 0.025 mg/ml) were added to the culture media at d 0 and the media were changed as described above for 8 d. The untreated cells consisted of identical media without ZnC. Cell extracts were prepared by adding a protein extraction solution (iNtRON Biotechnology). The lysates were clarified by centrifugation at 28,000×g for 20 min at 4°C, and the protein content of the supernatants was determined using a modified Bradford assay. The protein samples (30 μg) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose transfer membranes (Protran; Whatman, Germany). The membranes were blocked with 5% skim milk and probed with the following primary antibodies (dilution 1:1,000): C/EBPα (Santa Cruz, CA, USA), PPARγ (Santa Cruz, CA, USA), and β-actin (Santa Cruz, CA, USA). Specific proteins were identified by further incubation of the membranes with horseradish peroxidase-conjugated secondary antibodies (1:5,000) followed by a treatment with an enhanced chemiluminescence reagent (T&I, Korea) for 5 min and exposed to radiographic film (Kodak) for 1 to 10 min.
Statistical analysis
All quantitative data are representative of at least three independent experiments. Quantitative data are presented as the mean±standard deviation (SD), and Tukey’s post hoc least significant difference test was used to determine significance of differences among means using SAS 9.2 statistical software (Carolina, USA).
RESULTS AND DISCUSSION
Thermogravimetric analysis and differential scanning calorimetry
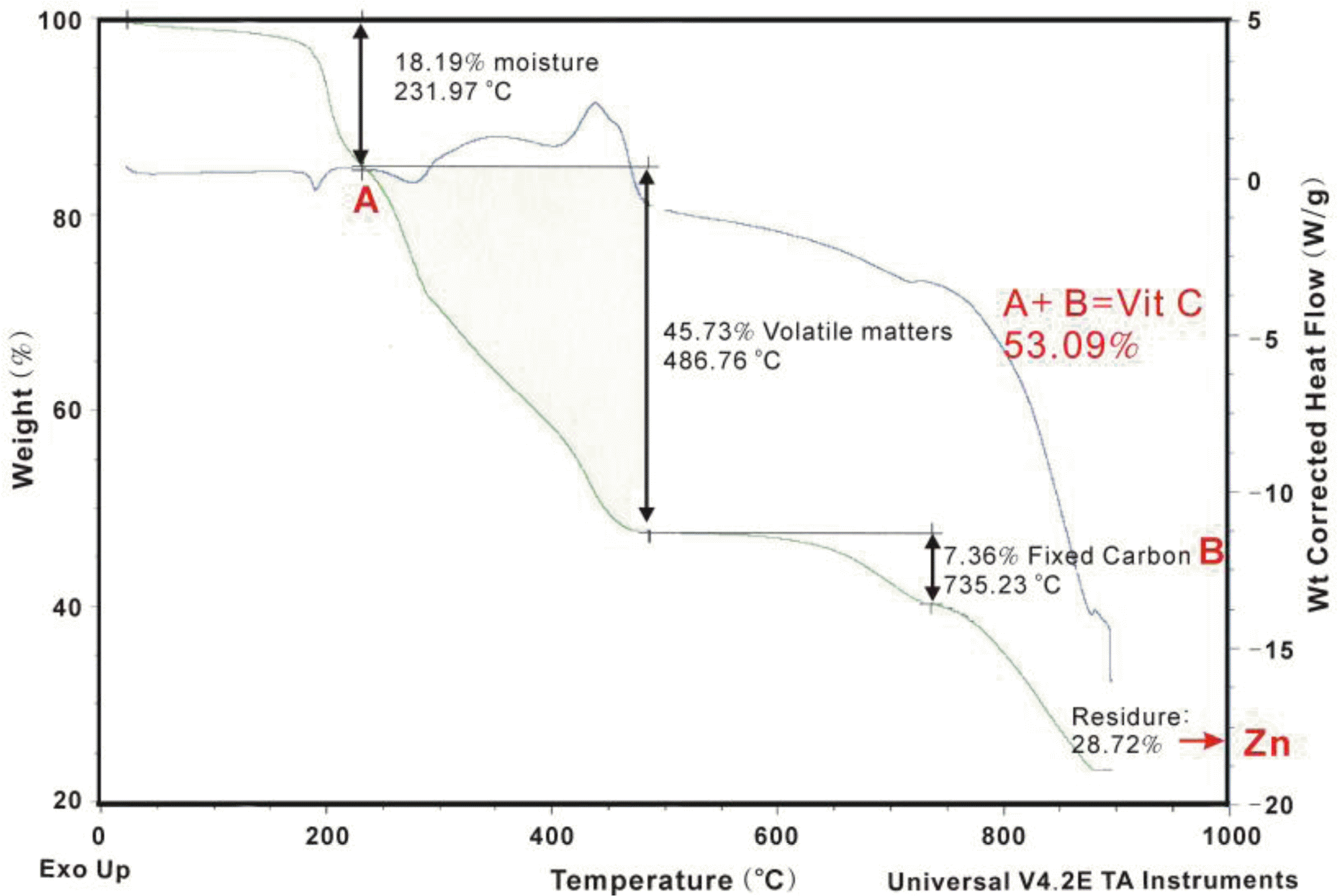
The thermogravimetric mass loss curve of the compound showed a multi-step decomposition profile (Figure 1). The first step, which proceeded rapidly and decayed up to 200°C, is the dehydration step. More than half of the total weight loss due to dehydration (18.2%) was observed below 200°C (5.3% at 95°C). The loss of organic matter from the ascorbate immediately occurred at elevated temperatures (Ünaleroǧlu et al., 2002). This indicates that some of the water molecules are involved in the outer coordination sphere. Zinc and vitamin C concentrations merged at 28.7% and 53.1%, respectively. Zinc and vitamin C were combined at a 1:2 ratio (Figure 2).
3T3-L1 cells are viable upon zinc-chelated vitamin C treatment
To investigate the potential effects of ZnC on the viability and cytotoxicity of 3T3-L1 cells in the early stage of adipocyte differentiation, differentiating 3T3-L1 cells were treated with different concentrations of ZnC (0, 0.012, and 0.025 mg/ml) for 24 and 48 h. ZnC treatment did not change the number of viable adipocytes appreciably (Figure 3). We can conclude that ZnC has no cytotoxic effect on cell viability.
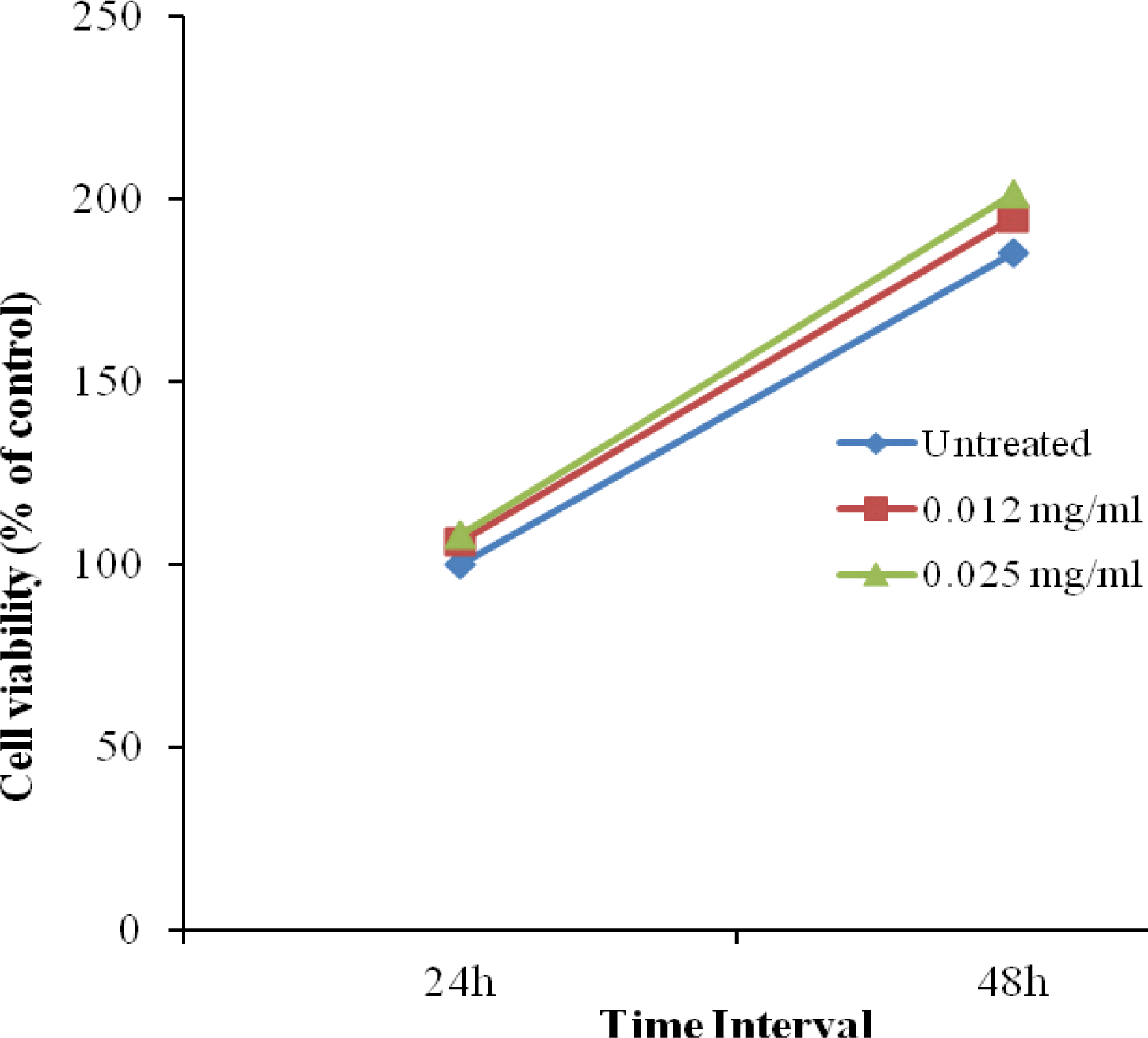
Effect of ZnC on the viability of differentiating adipocytes. Two day post-confluent 3T3-L1 cells were incubated for 24 and 48 h with an adipogenic cocktail and various concentrations of ZnC (0, 0.012, and 0.025 mg/ml). Cell viability was monitored using a CCK-8 assay after 24 and 48 h of treatment. Values are expressed as the mean±SD of three independent experiments.
Zinc-chelated vitamin C promotes lipid accumulation in 3T3-L1 cells
The representative images of Oil Red O staining demonstrated that ZnC promotes lipid accumulation. Adipogenesis is characterized by the successive activation of transcription factors and their downstream targets, resulting in lipid accumulation (Spiegelman and Flier, 2001); therefore, we investigated the effect of ZnC on the differentiation of 3T3-L1 cells. Morphological observations of cells stained with Oil Red O, a lipid stain, and OD values measured from isopropanol elution showed a significant increase in cellular lipid content with increased ZnC concentrations (Figure 4A and B). Furthermore, GPDH activity also supported this finding (Figure 5); the GPDH activity of all cultures treated with ZnC was enhanced in a dose-dependent manner.

Representative microscopic morphological images of adipocytes stained with Oil Red O (A) and the OD values measured from isopropanol elution of Oil Red O staining (B) after 8 d of differentiation. Preadipocytes were cultured in growth medium until they reached confluence and the quiescent cells were then incubated in differentiation medium (DM) and post-DM with 0, 0.012, and 0.025 mg/ml ZnC. At d 8 post-induction, Oil Red O staining was performed and images were taken at 10× magnification. Finally, the cells were destained with isopropanol and the OD of the destaining isopropanol was measured using spectrophotometry at a wavelength of 510 nm. Values are expressed as the mean±SD of three independent experiments. Bars within the same panel with different letters are significantly different (p<0.001).

GPDH activity of the adipocytes on d 8 of differentiation. Preadipocytes were cultured in growth medium until they reached confluence and the quiescent cells were then incubated in DM and post-DM with 0, 0.012, and 0.025 mg/ml ZnC. At d 8 post-induction, proteins were collected and GPDH analysis was performed. Values are expressed as the mean±SD of three independent experiments. Bars within the same panel with different letters are significantly different (p<0.001).
Zinc-chelated vitamin C induces the expression of adipogenic genes and promotes glucose uptake in adipocytes
Adipogenesis is governed by a complex series of transcriptional cascades that involve C/EBPs and PPARγ, which are considered to be the key regulators of adipogenesis. C/EBPα and PPARγ act synergistically to mediate the regulation of several genes that are responsible for the adipose phenotype and also glucose and lipid metabolism (Gregoire et al., 1998; Rosen, 2005). As ZnC promoted adipocyte differentiation, we observed the changes in the expression of C/EBPα and PPARγ using qPCR. ZnC significantly increased the mRNA levels of C/EBPα and PPARγ (Figure 6A and B). ZnC markedly induced the expression of aP2, a target gene of PPARγ and a marker of late differentiation in adipocytes (Figure 6C). C/EBPα plays a significant role in the regulation of GLUT4 and other genes involved in the metabolic actions of insulin (Wu et al., 1999). ZnC also significantly induced the expression of GLUT4, which serves a vital role in insulin-mediated glucose transport (Figure 6D), suggesting that ZnC treatment can enhance glucose uptake in differentiating adipocytes.
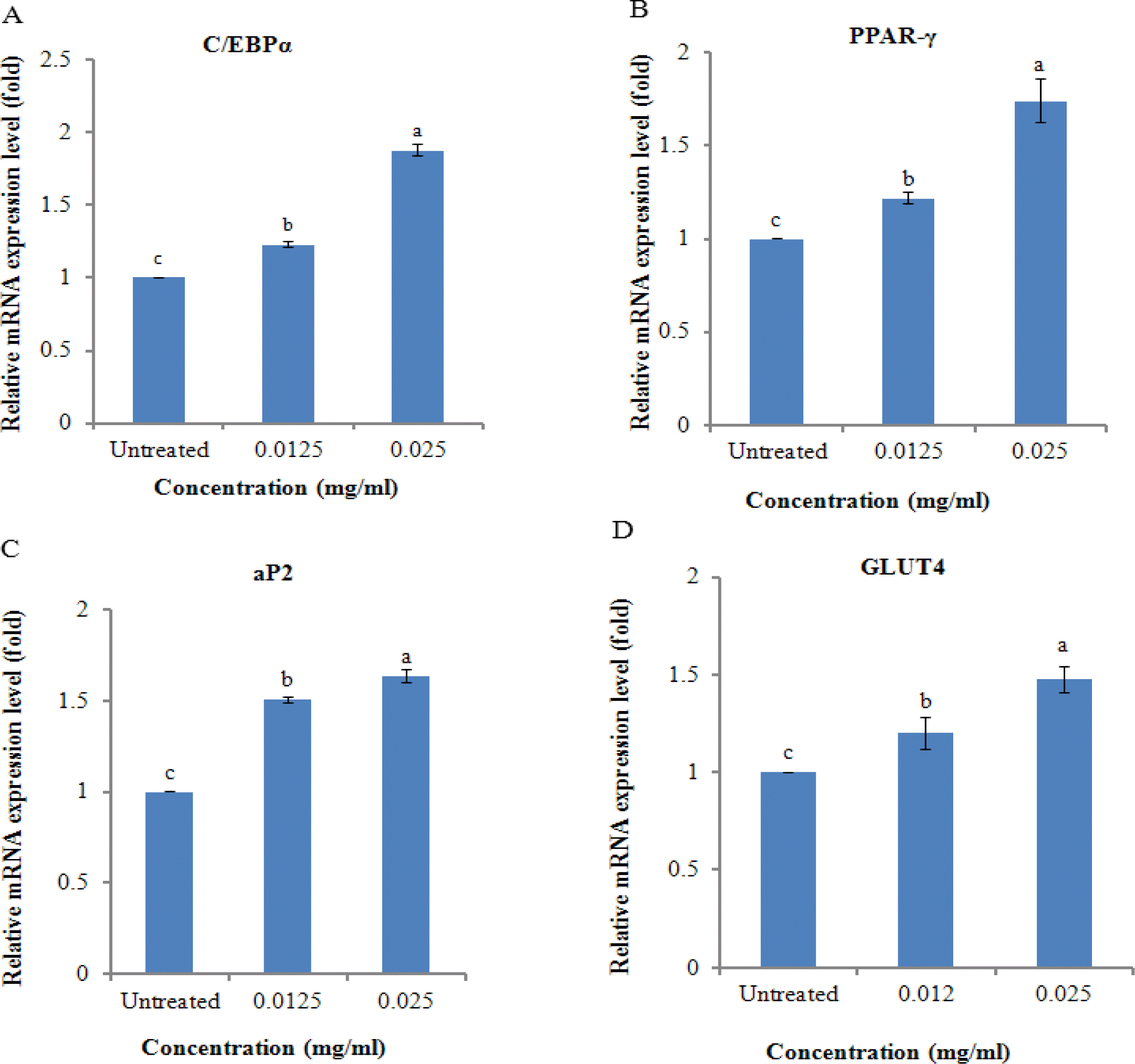
Effects of ZnC on the expression of genes associated with adipocyte differentiation. Post-confluent 3T3-L1 preadipocytes (d 0) were treated with different concentration of ZnC (0, 0.012, and 0.025 mg/ml) every 2 d for 8 days. On d 8, total cellular RNA was isolated from 3T3-L1 cells. The expression levels of (A) C/EBPα, (B) PPARγ, (C) aP2, and (D) GLUT4 were quantified by qPCR. Gene expression was normalized using β-actin as an internal control. Each bar represents the mean±SD of three independent experiments. Bars within the same panel with different letters are significantly different (p<0.001).
Zinc-chelated vitamin C enhances the protein expression of C/EBPα and PPARγ
C/EBPα and PPARγ are considered to be the most critical factors for adipogenesis. Generally, most of the transcriptional regulators of adipogenesis operate in a “feed-forward” fashion, whereby they induce other pro-adipogenic factors and then act in concert with those factors to promote downstream gene expression. PPARγ initially induces the expression of the adipogenic transcription factor C/EBPα and then binds with C/EBPα to the promoter/enhancer of the gene encoding aP2 (Green and Kehinde, 1976; Ross et al., 1990; Rosen, 2005). PPARγ and C/EBPα act synergistically to generate fully differentiated, insulin-responsive adipocytes (Tontonoz et al., 1994; Wu et al., 1999). Previous studies reported that zinc possesses insulin like action and Vit C promotes adipogenesis via increasing the growth rate and inducing differentiation in 3T3-L1 cells (May and Contoreggi, 1982; Kawada et al., 1990). As shown in Figure 7A and B, the protein expression of C/EBPα and PPARγ was increased in a dose-dependent manner by ZnC, suggesting that ZnC promotes adipogenesis in 3T3-L1 adipocytes through a C/EBPα- and PPARγ-dependent pathway.
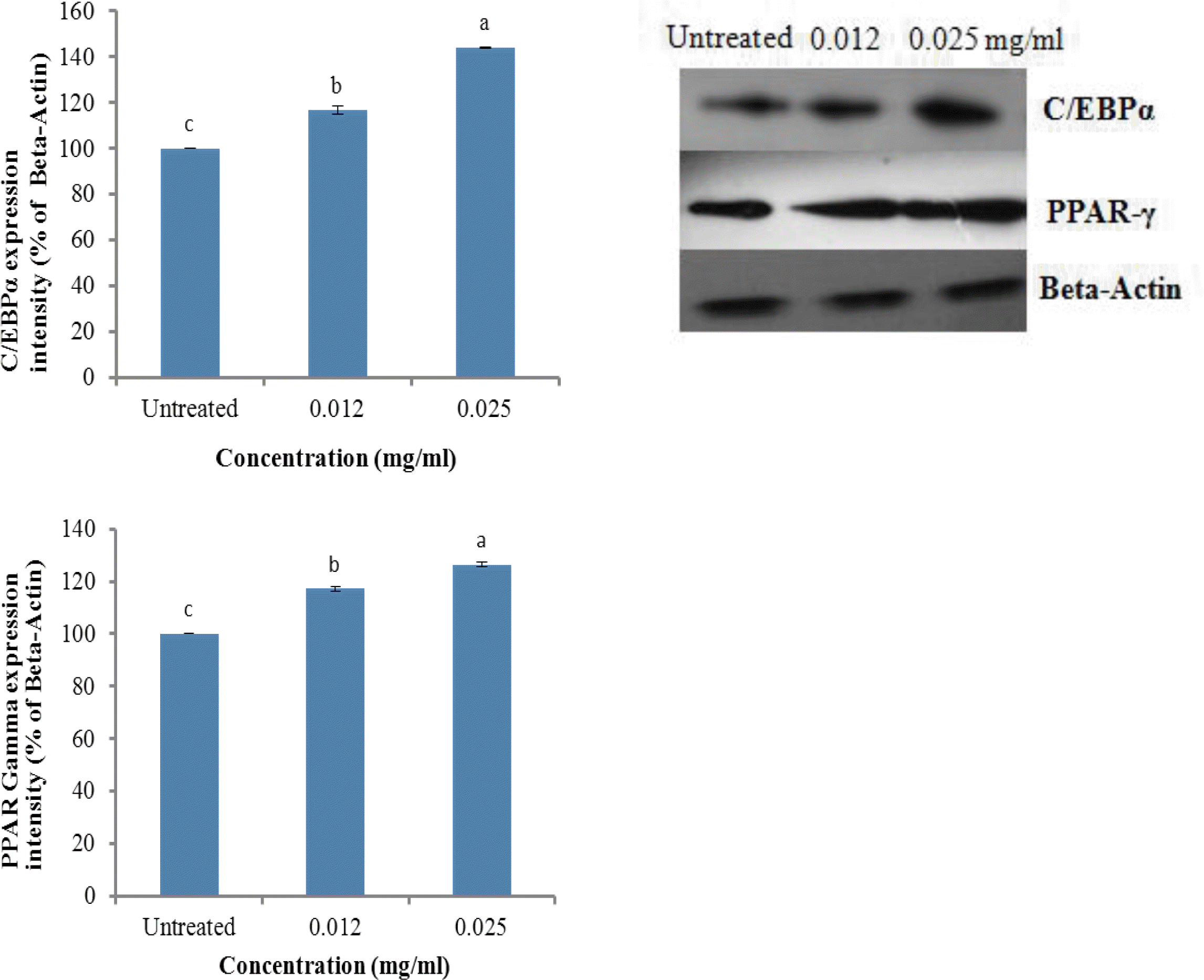
The effects of ZnC on the protein expression of C/EBPα (A) and PPARγ (B) in 3T3-L1 cells measured by western blot analysis. Preadipocytes were cultured in growth medium until they reached confluence and the quiescent cells were then incubated in DM and post-DM with 0, 0.012, and 0.025 mg/ml of ZnC for 8 d, and then harvested. An equal amount (30 μg) of protein from each sample was subjected to western blot analysis using antibodies for C/EBPα and PPARγ. Each bar represents the mean±SD of three independent experiments. Bars within the same panel with different letters are significantly different (p<0.001).
CONCLUSION
In the present study, we demonstrated that ZnC significantly promoted the adipogenesis of 3T3-L1 preadipocytes by enhancing the expression of the key transcription factors C/EBPα and PPARγ and the related adipogenic target gene aP2. Moreover, ZnC also stimulated the expression of GLUT4, a key regulator of insulin-mediated glucose transport in adipocytes. Taken together, these findings are helpful in understanding the insulin sensitizing and adipogenic properties of ZnC, which can be used as a novel therapeutic agent for the management of diabetes and related metabolic disorders. However, further studies are needed to assess the specific mechanism underlying the observed effects of ZnC.
Acknowledgements
This study was supported by a research grant from Hankyong National University for an academic exchange program in 2010.