Production, Nutritional Quality and In vitro Methane Production from Andropogon gayanus Grass Harvested at Different Maturities and Preserved as Hay or Silage
Article information
Abstract
Andropogon gayanus is an important grass due to its high biomass production, drought tolerance and favorable growth on low fertility acidic soils. Currently, there is little research on the impact of growth stage on the nutritional quality or the degree of CH4 production that may arise from this forage during ruminal fermentation. The objectives of this study were to determine the effects of regrowth stage of A. gayanus on its chemical composition, in vitro production of gas and CH4, as well as in vitro dry matter (DM) digestibility when grown under tropical Brazilian conditions and conserved as hay or as silage. The nutritional value of A. gayanus grass declined with increasing maturity; however digestible DM yield linearly increased. After 112 d of regrowth, A. gayanus produced higher quality silage (higher lactate and lower pH and butyrate content) and higher DM yield. However, the low levels of crude protein at this time would make protein supplementation a necessity for proper rumen fermentation. No differences in CH4 kinetic parameters were found with advancing maturity or preservation method (hay or silage).
INTRODUCTION
The edaphoclimatic conditions in Brazil favor the cultivation of tropical forage grasses. Andropogon gayanus is an important tropical grass due to its high biomass production and its ability to tolerate long dry seasons and low fertility, acidic soils. It is one of the most widely adapted grasses to the tropical savannah (CIAT, 1990) and has been extensively used for grazing ruminants in Brazil (mainly in Cerrado biome). South America has approximately 240 million ha of savannah and represents a major resource for cattle production on the continent (Lascano, 1991; CIAT, 1992). Tropical savannas are characterized by a climate with alternating wet and dry seasons (Cochrane, 1986). During the dry season there is scarcity of forage and when combined with a significant reduction in forage quality, grazing ruminants are often in negative energy balance. One management approach to avoid this problem is to ensile the surplus forage during the rainy season when quality is substantially higher.
It is well known that vegetative pastures have higher nutritional quality than mature pastures, but they also have lower dry matter (DM) yield and high moisture content. These properties can make ensiling challenging as they can lead to secondary fermentation, production of silage effluents and spoilage of the silage (McDonald et al., 1991). At more advanced maturity, DM yield is higher and the DM content (30 to 35% DM) is more favorable for silage production, but the high cell wall and lignin content can limit its consumption and nutritional value (Van Soest, 1994).
The stage of maturity of the plant can also influence digestive efficiency and therefore, enteric CH4 emissions. Johnson and Johnson (1995) surveyed the literature and confirmed that fermentation of fibrous carbohydrates leads to greater CH4 production as compared to non-fibrous carbohydrates. With advancing plant maturity, soluble carbohydrates decrease and lignification of plant cell walls increases, characteristics that promote the production of acetate and reduce the production of propionate in the rumen, thereby increasing the amount of CH4 produced per unit of forage digested (Robertson and Waghorn, 2002; Pinares-Patiño et al., 2007). Purcell et al. (2011) working with perennial ryegrass, observed that with advancing maturity, the digestibility of the herbage declined and this was paralleled by a reduction in CH4 per g DM incubated, but an increase in CH4 per g DM digested in vitro. According to Beauchemin et al. (2008) improving forage quality could contribute to a reduction in CH4 emissions per unit of forage digested, but this relationship is not well documented. Nonetheless, improvements in forage quality are believed to lower lifetime emissions or emissions per unit of animal product as a result of enhanced animal productivity (O’Mara et al., 2008). The relative scarcity of data on CH4 production from ruminants fed tropical forages (Archimède et al., 2011) at various stages of maturity (Meale et al., 2012) suggests a need for additional studies.
Harvest of grasses for silage production should occur in the vegetative stage when the plant is at a balance between appropriate DM yield and nutritional quality. Currently, there is little research assessing the nutritional quality of A. gayanus grass or the optimal time for ensiling with increasing days of regrowth. The objectives of this study were to determine the effects of advancing maturity of A. gayanus either fresh or as silage, on its nutritional value for ruminants as assessed by its chemical composition, in vitro gas and CH4 production as well as in vitro DM digestibility.
MATERIALS AND METHODS
Location and soil characteristics
The experiment was conducted in the rainy season of 2006 to 2007 at 19°35″36S, 43°51′56″W; altitude 747 m, using an established pasture of A. gayanus in Lagoa Santa County, Minas Gerais, Brazil (Viana et al., 2002). Rainfall, temperature and relative humidity data during the periods of herbage growth were obtained from local weather stations (in Belo Horizonte, 30 km from experimental area) and are shown in Figure 1. Soil samples were collected at a depth of 0 to 20 cm prior to the commencement of the experiment and these analyses are presented in Table 1. To neutralize acidity, lime was applied (2,000 kg/ha) at the beginning of the rainy season. After 30 d the grass was cut 20 cm above the soil level and 250 kg/ha of 08-24-12 and 100 kg/ha of 30-00-20 (N:P:K) was applied.
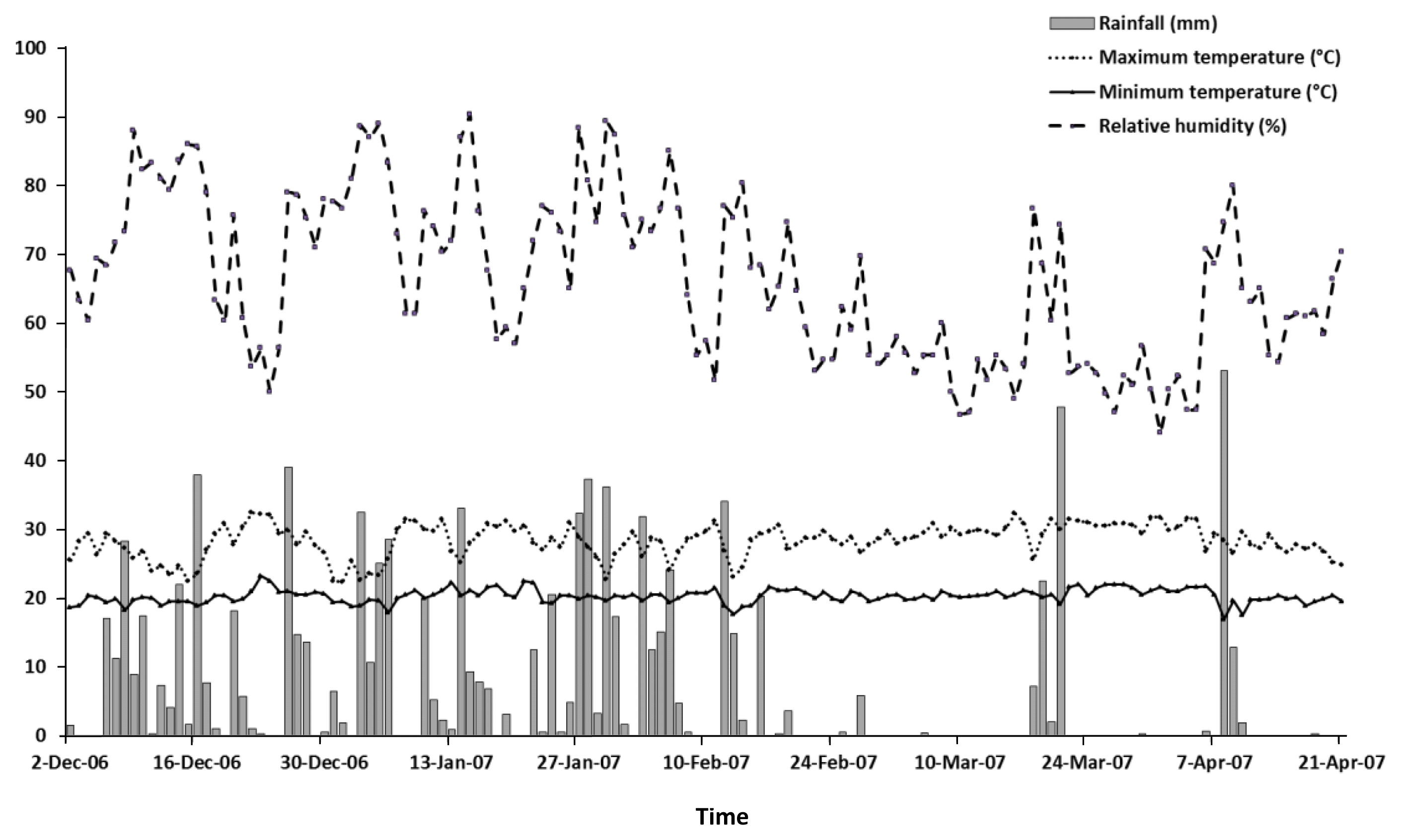
Rainfall, minimum and maximum temperature and relative humidity from December 2006 to April 2007 at the experimental station of Belo Horizonte, MG, Brazil.
Treatments
Andropogon gayanus grass was harvested at different days of regrowth (56, 84, 112, or 140 d). At the beginning of the experiment, the experimental area was divided into five sub-areas (blocks) and four plots (8×8 m) were established in each sub-area (20 plots total). Five random plots (one in each sub-area) were harvested at each regrowth age, weighed and chopped (10 to 25 mm). Before harvest, height, leaf area index, light interception and leaf angle were measured using a plant canopy analyzer (LAI2000; LI-COR, Lincoln, Nebraska, USA). A subsample from each plot was frozen at −20°C, and the remainder was immediately ensiled in polyvinyl chloride laboratory silos (40 cm long and 10 cm diameter). The silos were closed with polyvinyl chloride lids equipped with a Bunsen valve. Two silos were made per plot and after 56 d, both silos were opened and silage between replicates pooled. At this time, samples were divided in two equal portions. For the first portion, extracts of fresh silage were obtained with a hydraulic press and immediately analyzed for ammonia nitrogen. Silage extracts (10 mL) were also taken, acidified with 2 mL of metaphosphoric acid (0.25; w/v) and frozen at −20°C until analyzed for acetic, propionic, butyric and lactic acid. The second portion of silage was dried at 55°C for 72 h in a forced air oven. Fresh grass samples were thawed and dried as described above. All samples [n = 40 (5 plots×4 ages×2 treatments (grass or silage)] were ground through a 1 mm screen using a Wiley mill and used to for measurements of in vitro gas production, DM digestibility and chemical analysis.
Chemical analysis
Dry matter was determined by drying at 105°C for 12 h followed by equilibration in a desiccator (AOAC, 1990, ID 934.01), and organic matter (OM) was calculated as weight loss upon ignition at 600°C. The neutral detergent fiber (NDF) and acid detergent fiber (ADF) contents were determined by the sequential method with the ANKOM fibre analyzer using reagents as described by Van Soest et al. (1991) and expressed exclusive of ash. Sodium sulfite and α-amylase were used during NDF determination. Lignin analyses were performed on ADF residues, using the direct sulphuric acid method (Robertson and Van Soest, 1981). Hemicellulose was determined by subtracting ADF from NDF and cellulose was calculated as ADF minus lignin. Crude protein (CP) was determined by the Kjeldahl method (AOAC, 1990, ID 990.03) and water soluble carbohydrates (WSC) according to the methodology of Bailey (1967). Ammonia-N in silage extracts was determined by the Kjeldahl method with the volatile N fraction generated by heating the solution at pH >7 in the presence of MgO. Organic acids in silage extracts were analyzed using a gas chromatograph (GC-17 Shimadzu gas chromatograph) equipped with a flame ionization detector and fitted with a Nukol capillary column according to the methodology described by Playne (1985). The gas chromatograph was operated isothermally with a column temperature of 200°C and an inlet and detector temperature of 225°C.
In vitro DM digestibility
All procedures and protocols used in this experiment were approved by the Lethbridge Research Centre Animal Care Committee in accordance with the Canadian Council on Animal Care guidelines. The in vitro DM digestibility (IVDMD) was performed according to the two-stage procedure described by Tilley and Terry (1963), adapted by Holden (1999) using the rumen simulator DaisyII ANKOM system. Briefly, ANKOM bags (model F57) were rinsed with acetone and dried in a forced air oven at 60°C for 6 h before use. Ground samples (0.5 g/bag) were weighed into duplicate bags, heat sealed and placed in digestion jars. Rumen fluid was collected 2 h after feeding from two ruminally cannulated Holstein cows fed a mixed diet consisting of 70% tifton hay, 27% concentrate (corn and soybean meal based with 20% CP) and 3% supplement. Rumen fluid from each cow was combined and incubated with the samples for 48 h. At this point, 40-mL of 6 N HCl and 8 g of pepsin powder were added to each digestion vessel and incubation continued for a further 24 h. Upon completion, jars were removed from the chamber, the solution was discarded, and bags were rinsed with distilled water. Bags were dried at 105°C for 24 h and weighed.
In vitro gas production
For each in vitro incubation, 0.5 g DM of sample was weighed into an ANKOM bag (model F57) with 2 replicates/field plot/treatment (grass or silage with different regrowth ages) and sealed. Each bag was placed into a 125 mL amber serum bottle fitted with rubber stoppers. The entire incubation was repeated (i.e., 2 incubation runs×2 replicates×5 field plot/treatment), resulting in a total of 20 replicate vials/treatment (grass or silage at different regrowth ages).
Inoculum for the in vitro incubation was obtained from three ruminally cannulated Holstein cows fed a mixed diet consisting of 27% rolled barley grain, 3% supplement and 70% barley silage. Rumen fluid was collected 2 h after feeding from four distinct sites in the rumen, filtered through four layers of cheesecloth, combined in equal portions and transported in a prewarmed Thermos flask to the laboratory. Inoculum was prepared by mixing rumen fluid and a mineral buffer with 0.5 mL of cysteine sulphide solution (Menke et al., 1979) in a ratio of 1:2. The inoculum was then transferred (40 mL) into pre-loaded prewarmed (39°C) vials under a stream of O2-free N gas. Vials were sealed and placed on an orbital shaker set at 90 oscillations/min in an incubator at 39°C.
Net gas production of each vial was measured after 6, 12, 24, 36, 48, and 72 h of incubation using a water displacement apparatus (Fedorak and Hrudey, 1983). Prior to gas measurement, 10 mL of headspace gas was sampled using a 20 mL syringe from one replicate vial from plots 1 and 2 in the first run, and from plots 3, 4, and 5 in the second run. Gas was immediately transferred into a 5.9 mL evacuated Exetainer (Labco Ltd., High Wycombe, Buckinghamshire, UK). Collected gas was analyzed for CH4 concentration by gas chromatography as described by Holtshausen et al. (2009). Methane was expressed as mL of CH4/g of DM incubated and per g of DM disappeared after 72 h of incubation. Total net gas production was reported as mL/g of DM incubated. The pH of the culture was measured using a pH meter (Orion Model 260A, Fisher Scientific, Toronto, ON, Canada). The ANKOM bags with the residues were then removed from the bottles, rinsed thoroughly with distilled water, pre-dried at 55°C for 48 h and dried at 105°C for 24 h to a constant weight and weighed to estimate apparent DM disappearance (aDMD). The bags were also analysed for NDF as described above to estimate NDF disappearance (NDFD).
The liquid fraction of the fermentation was subsampled at the beginning and end of the incubation for determination of ammonia and volatile fatty acids (VFA). One sample (1.5 mL) from each vial was transferred to microcentrifuge tubes (2 mL) containing 300 μL of H2SO4 (0.01; v/v) and centrifuged at 14,000×g for 10 min at 4°C (Spectrafuse 16 M, National Labnet Co., Edison, NJ, USA) to precipitate particulate matter and protein. The supernatant was transferred into microcentrifuge tubes (Fisher Scientific, Ottawa, ON, Canada) and frozen at −20°C until analyzed for ammonia N.
Another subsample (1.5 mL) was collected, acidified with 300 μL of metaphosphoric acid (0.25; w/v) and centrifuged as described above for ammonia N analysis. The supernatant was frozen at −20°C until analyzed for VFA. The 0 h samples were also analyzed for ammonia N and VFA to calculate net ammonia-N and total VFA production (Holtshausen et al., 2009).
Gas kinetics and statistical analyses
Gas production (GP) and CH4 data were fitted to the non-linear model proposed by López et al. (1999):
Where, B is the asymptotic gas production (mL/g DM incubated), k is the fractional fermentation rate (h−1), L is the lag time (h), and t the incubation time (h). An iterative least squares procedure using NLIN (SAS Institute, Inc. 2013; version 9.2) was used to estimate B, k, and L. Average fermentation rate (AFR, mL gas/h) was defined as:
Agronomic characteristics and silage fermentation characteristics were analyzed as a completely randomized block design for AGE affect using the PROC MIXED procedure of SAS (SAS Institute, Inc. 2013). Chemical composition data and in vitro gas production parameters were analyzed as a factorial design for AGE, SAMPLE TYPE (hay×silage) and AGE×SAMPLE TYPE using the PROC MIXED procedure of SAS. When there was an effect (p<0.05) of AGE, orthogonal polynomials were performed to determine if stage of maturity resulted in a linear or quadratic effect on measured parameters. Differences were considered significant when p<0.05.
RESULTS
Agronomic characteristics
Dry matter yield and digestible DM yield increased quadratically (p<0.05) with increasing grass maturity (Table 2). Pasture height increased quadratically (p<0.05) with increasing proportion of and the proportion of leaves linearly decreasing (p<0.01) with increasing grass maturity. Sward light interception increased quadratically (p<0.05) and leaf area index increased linearly (p<0.05) with age of regrowth.
Chemical composition
There was no interaction (p>0.05) between sample type (hay×silage) and regrowth age for DM, OM, CP, NDF, ADF, lignin, hemicellulose or IVDMD. However, an interaction (p<0.05) between sample type and regrowth age was observed for cellulose and WSC (Table 3). The DM, NDF and ADF increased and IVDMD decreased quadratically (p<0.05) and OM and hemicellulose linearly increased (p<0.05) while CP linearly decreased (p<0.05) with increasing maturity regardless of preservation method (Table 3). Lignin content did not change (p>0.05) in the hay or silage (p = 0.16) with increasing maturity. In addition, the content of cellulose linearly increased in hay (p = 0.02) and quadratically in silage (p<0.01) with increasing maturity of A. gayanus. Water soluble carbohydrate (WSC, g/kg DM) quadratically increased with increasing grass maturity in hay (p<0.05) and was not affected in silage (p>0.05) with all values being close to zero.
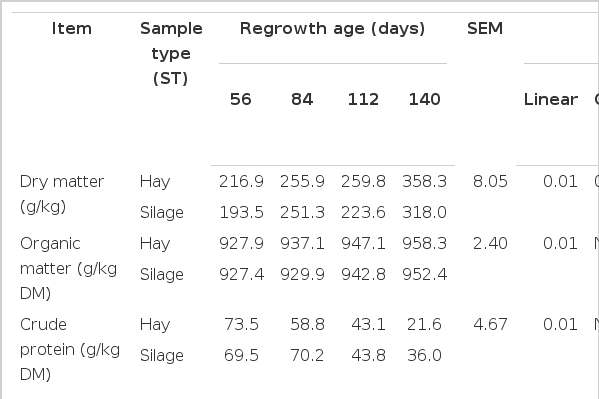
Chemical composition and in vitro dry matter digestibility (IVDMD) of Andropogon gayanus grass harvested at different regrowth stages and preserved as hay or silage
Ensiling promoted a reduction (p<0.01) in DM, OM, hemicellulose and WSC, which resulted in a reduction (p<0.01) in the IVDMD of silage as compared to hay (Table 3). The content of CP, NDF and lignin did not differ (p>0.05) between grass hay and silage, but ADF and cellulose increased in silage (p<0.05).
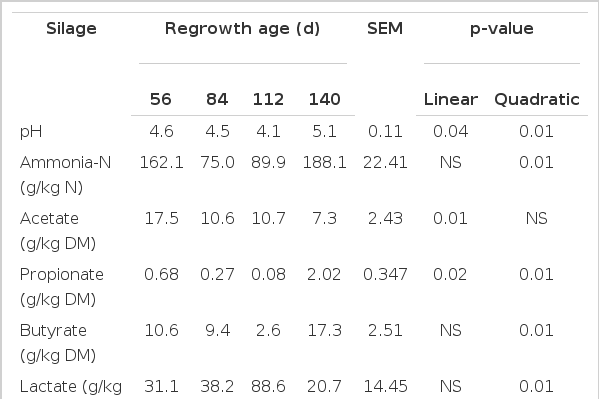
A quadratic effect was observed for silage pH (p = 0.01) with grass cut at 112 d of regrowth exhibiting the lowest pH (p<0.01; Table 4). A quadratic effect was also observed for ammonia-N content with lower values (p<0.01) in silages made with grass at the intermediate stage of regrowth (84 and 112 d) and higher (p<0.01) levels associated with the earliest and latest cuts (56 and 140 d). Silage acetate content decreased linearly (p<0.01; Table 4) with increasing grass maturity, but propionate and butyrate concentrations (g/kg DM) exhibited a quadratic response (p<0.01), with the lowest levels being encountered for the silage made using the grass cut after 112 d of regrowth. A quadratic effect (p = 0.02) was also observed for lactate in silage, with the highest concentrations being found in the silage made with grass after 112 d of regrowth (Table 4).
In vitro gas production
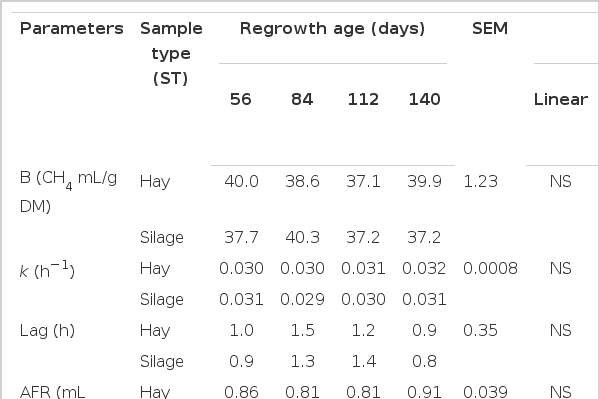
An interaction between sample type and regrowth age was observed (p<0.05) for the maximum potential of gas production (B) and fractional fermentation rate (k) (Table 5). Both hay and silage exhibited a linear decrease (p<0.05) in B with increasing grass maturity. Maximum gas production was not affected (p>0.05) by the method of preservation. A quadratic increase in the hay and a linear increase in the silage fractional fermentation rates (k) were observed with increasing regrowth. Preservation method affected the fractional fermentation rate (k), with values lower (p<0.05) for silage as compared to hay. An interaction (P>0.05) between sample type and regrowth age was not observed for lag time or average fermentation rate (AFR). Lag time was lower (p<0.01) for hay and silage produced from grass after 112 d of regrowth. A quadratic response (p<0.01) was apparent for AFR with the lowest values in grass harvested after 84 days of regrowth. Ensiling of the grass reduced (p<0.01) the AFR.

In vitro gas kinetic parameters, apparent dry matter disappearance (aDMD) and NDF disappearance (NDFD) after 72 h of incubation of Andropogon gayanus grass harvested at different regrowth stages and preserved as hay or silage
Total gas production per g of apparent DM disappearance (aDMD, after 72 h) in hay exhibited a quadratic pattern, being lower (p<0.01) for grass harvested after 84 and 112 d of regrowth and higher (p<0.01) for that harvested after 56 and 140 d. However, gas production (mL g/f aDMD) among silages showed a linear (p<0.01) increase with increasing grass maturity.
Grass maturity did not affect (p>0.10) aDMD or NDFD of hay, but these parameters were negatively affected (p<0.01) when the grass was ensiled (Table 5). The highest (p<0.01) DM and NDF disappearances were observed in silage made with grass after 112 d of regrowth, and the lowest (p<0.01) in silage from grass after 140 d of regrowth.
Total VFA concentration (mM), proportions of acetate, propionate, butyrate, BCVFA, valerate, caproate of total VFA and acetate/propionate ratio were not affected (p>0.05) by grass maturity (Table 6). Maturity affected ammonia-N concentrations (mmol) in a quadratic matter (p<0.05) with it being lower for grass harvested after 112 d of regrowth. The pH was not affected (p>0.05) by maturity in incubations with grass hay, however it was affected (p<0.01) in silage. Silage incubations linearly reduced pH (p<0.01) with increasing grass maturity.
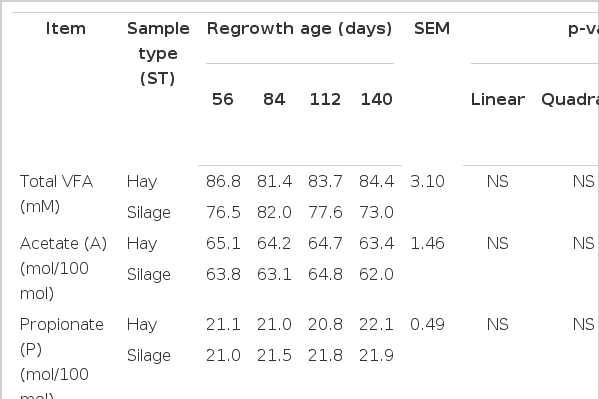
In vitro fermentation characteristics after 72 h of incubation of Andropogon gayanus grass harvested at different regrowth stages and preserved as hay or silage
Gas production g−1 of aDMD, Admd, and NDFD were higher (p<0.05) in grass hay as compared to silage (Table 5). Total VFA was also higher (p<0.01) in incubations with hay as compared to silage, resulting in a lower (p = 0.02) pH (Table 6). Acetate, propionate, butyrate, BCVFA, valerate, caproate, acetate/propionate ratio and ammonia N were not affected (p>0.05) by ensiling. In vitro CH4 kinetic parameters were not affected (p>0.05) by grass maturity or preservation method (Table 7).
DISCUSSION
Agronomic characteristics
Increasing DM and digestible DM yields with increasing grass maturity is consistent with other research on tropical grasses (Mandebvu et al., 1999; Dore, 2006). Adjolohoun et al. (2008) reported DM yields of 2,087 kg/ha for Panicum maximum cv. C1 and 1,528 kg/ha for Pennisetum purpureum after 45 d of regrowth in the tropical savanna of Benin. Sousa et al. (2010) working with Brachiaria brizantha cv. Marandu in the “Cerrado” biome of Brazil, observed DM yield of 1,100 kg/ha after 30 d of regrowth. Our results confirm the high biomass production potential of this grass in the tropical savannah. Together with the increase in DM yield, a quadratic increase (p<0.01) in sward height was also observed. The increase in the grass height and DM yield with increasing maturity was mainly due to stem elongation which is consistent with a reduction in leafiness with increasing maturity. Consequently, plant height is an excellent indicator of DM yield and nutritional quality and is easily measured on-farm as a guideline for pasture management.
Leaf area index increased up to 84 d of regrowth, a period in which light interception was 0.96. These results are in agreement with Mello and Pedreira (2004) in which leaf area index increased up to 0.95 of sward light interception and stabilized thereafter due to shading of the lower leaves.
Chemical composition
Maturity is considered to be the primary factor affecting the chemical composition and nutritional quality of most forages (Nelson and Moser, 1994). An increase in DM content with increasing maturity was expected. According to Demarquilly and Dulphy (1977) the optimal DM content for ensiling of forage is 270 to 380 g/kg in order to avoid seepage losses and the growth of spoilage microorganisms. In our study, only the forage ensiled after 140 d of regrowth exhibited a DM content within these limits. The nutritive value of forages linearly declines with increasing physiological maturity (Blaser et al., 1986). The increase in NDF and ADF and decrease in CP and IVDMD with increasing grass maturity observed in this study confirms this concept. Our results show that even after 56 d of regrowth the NDF (710 g/kg DM) and ADF (380 g/kg DM) of A. gayanus were very high. At these levels forage intake would likely be reduced as NDF concentrations is negatively correlated with voluntary intake (Van Soest, 1994).
The decrease in digestibility and CP of tropical forages is considered to be directly related to a higher proportion of stem in the plant. This was confirmed in our study as both CP and IVDMD decreased with an increase in the proportion of stems. It is also important to emphasize that the CP concentrations were lower than 60 g/kg DM after 112 and 140 d of regrowth, a level that is too low to sustain optimal activity by microbes for efficient ruminal fermentation (Minson, 1990; Van Soest, 1994). Therefore, if mature A. gayanus grass is to be grazed, it is important to include a protein supplement to ensure ruminal fermentation is not compromised.
Several studies have shown an increase in lignin content with increasing maturity of grasses (Blaser, 1964; Jung and Vogel, 1986; Jung and Vogel, 1992; Van Soest, 1994). In our study changes in lignin content were not observed with increasing grass maturity, however lignin levels were already high (>60 g/kg of DM) at the first harvest, a characteristic of this grass species. Consequently, factors other than an increase in lignin content such as a general thickening of plant cell walls must be responsible for the decline in digestibility with advancing maturity.
The increase in WSC with increasing maturity in this study is in agreement with other researchers (Smith, 1973; McGrath, 1988; Silva Jr., 2004). Environmental studies have also indicated that increasing light intensity tends to increase water soluble carbohydrate content in forages (Van Soest et al., 1978). The increase in light intensity as the experiment was conducted through summer may also have affected the level of WSC observed.
A decrease in DM and OM content with ensiling was expected due to substrate fermentation by plant enzymes and bacteria in the silo. The reduction in hemicellulose content encountered in this study could be expected with the low levels of WSC that are typical of tropical grasses. According to McDonald et al. (1991) hemicellulose may be used as an additional substrate when the availability of soluble carbohydrates limits silage fermentation. A difference in hemicellulose content among silages suggests that hemicellulose was utilized for fermentation more in the first than second regrowth stage. As hemicellulose constitutes a portion of NDF, it was expected that NDF would also decrease during fermentation, but this relationship was not observed.
The results show that there was almost complete fermentation of WSC when grass was ensiled. Water-soluble carbohydrates are the main substrate used by lactic acid bacteria during silage fermentation (McDonald et al., 1991). According to Gourley and Lusk (1978), 60 to 80 g/kg DM of soluble carbohydrates are required for efficient ensiling and the formation of good quality forage. The values observed in A. gayanus grass were all lower than this and may have limited ensiling at earlier stages of regrowth (56 and 84 d) as suggested by fermentation characteristics (pH, butyrate and lactate levels; Table 4). A reduction in IVDMD with ensiling is expected as the easily fermentable carbohydrates are utilized during the ensiling process.
The highest level of pH (5.07) and ammonia-N was observed in silage made using grass after 140 d of regrowth. These results are in agreement with others that observed high pH silage favors secondary fermentation, leading to an increase in ammonia-N (McDonald et al., 1991). Although grass ensiled after 140 d of regrowth had the highest WSC content, it also exhibited the highest pH. This may be due to high DM content of grass at this age, which could have impaired fermentation and limited pH decline (Muck, 1988). The high amount of ammonia-N in silage made with the grass after 56 d of regrowth is a reflection of its higher moisture and crude protein content at the time of harvest. According to Oshima and McDonald (1978) and Henderson (1993), ammonia-N levels in good quality silage should not be higher than 8 to 11% of total N. Only silages made with grass harvested after 84 and 112 d of regrowth were within this limit.
A reduction in butyrate production with advancing plant maturity was expected owing to an increase in the DM content of mature forage. However, in this work, the silage produced from grass after 140 d of regrowth showed the highest level of butyrate. This can be explained by the higher pH of this silage compared with other silages. The high silage pH favors the development of Clostridium spp., which are producers of butyrate, and reduce the nutritional value of silage (McDonald et al., 1991).
The silage produced from grass after 112 d of regrowth had the highest level of lactate and the lowest pH, whereas the silage made after 140 d of regrowth had the lowest lactate content and the highest pH. These results show the importance of lactate production in reducing silage pH. Despite the contribution of all acids formed during fermentation, lactate plays a critical role in the reduction of silage pH due to its high dissociation constant (Moisio and Heikonen, 1994).
According to Kung Jr. (2001) the optimal organic acids levels at the end of the fermentation process in tropical grasses silage should be 60 to 100 g/kg DM of lactate, 10 to 30 g/kg DM of acetate, less than 1 g/kg DM of propionate and 0 g/kg DM of butyrate. In this work the forge cut after 112 d regrowth produced silage with an acid content that fell within these ranges, with only butyric acid exceeding recommended levels.
In vitro gas production
A decrease in gas production with increasing maturity is to be expected due to a reduction in the plant cell wall degradability. This was observed in this study by the linear decrease in the maximum potential of gas production (B) with increasing grass maturity. Blümmel and Ørskov (1993) demonstrated that total gas production (B) was correlated with intake (0.88), digestible dry matter intake (0.93) and growth rate (0.95) in a multiple regression model. Inclusion of the rate of gas production (k) in the model did not improve the prediction of these relationships. The higher aDMD (after 72 h) and NDFD for the silage made with grass after 112 d of regrowth may be explained by an improvement in the fermentation process as reflected by the higher level of lactate in this silage. Although a linear decrease was observed in the silage batch culture pH with increasing grass maturity, all pH values were very close or above 6.6.
A higher level aDMD (after 72 h), NDFD and total VFA with hay compared to silage in this study was expected due to the consumption of easily fermented carbohydrates (WSC and hemicellulose) during the ensiling process. The lower pH encountered for hay is due to the higher total VFA production during in vitro fermentation. It is generally accepted that, the fermentation of fibrous carbohydrates leads to greater CH4 production as compared to non-fibrous carbohydrates (Johnson and Johnson, 1995). High soluble carbohydrate content is suggested to promote the production of propionate in the rumen, lower ruminal pH and inhibit methanogens thereby reducing CH4 production per unit of OM fermented (Van Kessel and Russell, 1996). Assoumaya (2007) compared digestion of the C4 plant Panicum maximum between 14 and 56 d of re-growth and found a decrease in propionate relative to total VFA, while acetate increased with advancing maturity. Lower CH4 production (mL/g of DM incubated and/or mL/g of DM digested) with more immature swards was expected as the lower fibre content shifts fermentation towards production of propionate. In contrast, increases in fiber contents of forages with increasing maturity promotes acetate production, making more hydrogen available for methanogenesis and resulting in higher CH4 production. However, in this work in vitro CH4 production (mL/g of DM incubated and/or mL/g of DM digested) was not affected by maturity with either hay or silage. The results are in agreement with the total VFA profiles which also did not differ with maturity for either hay or silage. According to Navarro-Villa et al. (2011) the use of more immature swards (perennial ryegrass) increased herbage digestibility and resulted in higher VFA output and CH4/g DM incubated, but when CH4 was expressed per g DM digested no difference was found. However, research with perennial ryegrass by Purcell et al. (2011) observed that with advancing maturity, the digestibility of the herbage declined and that this was accompanied by a reduction in CH4 output/g DM incubated, but an increase in CH4 output/g DM digested. According to Purcell et al. (2011) the decreases in WSC and CP, and increases in aNDFom and ADFom that occur as grass matures, combined with a small linear increase in pH, created conditions that promoted the activity of cellulolytic bacteria. This resulted in a linear increase in the proportions of acetic and butyric acid, and a decrease in propionic acid, increasing methanogenesis. The differences in chemical composition with maturation found in work by Purcell et al. (2011) was greater than we observed, a factor that may account for the lack of difference in CH4 production among forage at different stages of regrowth in our study. In this work, the immature A. gayanus sward (56 d of regrowth) already had high NDF concentrations (720 g/kg DM) and differed only marginally from the mature sward (752 g/kg DM). The lack of an effect of grass maturity on in vitro rumen total VFA concentration, the proportions of VFA, the A:P ratio, and consequently CH4 production (mL/g of DM incubated and mL/g of DM digested) was not surprising considering the relatively small effect of the regrowth stage on the chemical composition of the forage.
It is widely accepted that feedstuffs which have higher gas production and IVDMD tend to have higher CH4 production per g DM incubated (Durmic et al., 2010; Njidda and Nasiru, 2010; Jayanegara et al., 2011). Therefore we anticipated observing higher CH4 production (mL/g of DM incubated) for hay compared to silage, but CH4 kinetic data did not show such a relationship. Cushnahan et al. (1995) attributed the higher CH4 output per unit gross energy intake in vivo for grass as compared to silage to increased intake and WSC concentration of grass. Navarro-Villa et al. (2012) also observed that ensiling of perennial ryegrass (Lolium perenne L., cv. Greengold) reduced CH4 production (mL/g of DM incubated and/or mL/g of DM digested) in 24 h in vitro incubations. The authors attributed the differences to a reduced total gas production and a greater molar proportion of propionic acid for silage as compared to fresh grass.
CONCLUSIONS
Results from this study show that the nutritional value of A. gayanus grass was reduced with increasing maturity, however, the digestible DM yield quadratically increased with maturation. Andropogon gayanus grass after 112 d of regrowth produced better quality silage and higher DM yield. Nevertheless, the low level of crude protein at this stage of maturity would make it essential to supplement the diet with protein for proper rumen fermentation. Finally, neither differences in CH4 production nor CH4 kinetics were found when this grass was preserved as either hay or silage over a range of maturities.
ACKNOWLEDGEMENTS
This study was conducted with funding from FAPEMIG - Minas Gerais State Research Foundation (process CAG - 2653/06) and Alberta Meat and Livestock Agency (ALMA). The first author gratefully acknowledged CNPq - the Brazilian National Council for Scientific and Technological Development for the doctorate sandwich scholarship. The authors also thank K. Munns, W. Smart, D. Vedres, F. Van Herk, M. He and Y, Wang of the Lethbridge Research Centre, AB, Canada for their contributions to this study.