 |
 |
Abstract
The present investigation was designed to determine whether supplementation of different level of vitamin E for 12 months to arsenic exposed goats (50 ppm as sodium arsenite) affords protection against the blood hemato-biochemical parameters caused by the metalloid. A total of 24 crossbred (Alpine×Beetal) lactating goats were assigned randomly into 4 equal groups (control, T1, T2 and T3) of 6 in each, on the basis of average body weight (36.10±0.11 kg) and milk yield (1.61±0.04 kg/d). The animals in T1, T2 and T3 were given 50 ppm arsenic, while in T2 and T3, additionally; vitamin E at the rate of 100 IU and 150 IU/kg dry matter (DM) respectively was additionally supplemented for the period of 12 months. Hemoglobin (Hb), total leukocyte (TLC) and blood lymphocyte % were decreased (p<0.05) in arsenic fed groups and vitamin E supplementation in the experimental group showed a protective potential. Significant increases (p<0.05) in aspertate transaminase (AST) and alanine transaminase (ALT) activities among arsenic supplemented groups were recorded, however vitamin E supplementation at higher doses showed a protective effect (p<0.05) against AST but in the case of ALT no ameliorating effect was found in either of the doses. Plasma total protein was decreased (p>0.05) but creatinine level was periodically increased in all As supplemented groups and vitamin E supplementation did not produce any protective effect. It can be concluded that arsenic exposure resulted in varying degree of changes in hemato-biochemical parameters and activities of antioxidant enzymes in goats but concomitant treatment with Vitamin E is partially helpful in reducing the burden of arsenic induced effect.
Arsenic (As) is a metalloid found in water, soil, and air from natural and anthropogenic sources (Hughes, 2002). Arsenic occurs in both organic and inorganic forms in nature, but the inorganic form is more toxic and represents a potential threat to the environment, human health, and animal health. Various livestock animals are also the likely victims of such catastrophes arising from As pollution due to contamination of drinking water. Administration of As could affect animal performances and serum clinical parameters. Liver and kidneys are considered as the primary targets for its toxico-pathological manifestations and there are a few reports of biochemical alterations indicative of hepatic and renal system involvement in As toxicity in animals (Biswas et al., 1998; Santra et al., 1999; Nandi et al., 2005). However, little data have been reported in lactating dairy animals. For cattle, sheep and swine maximum tolerable dietary As recommended is 50 g/kg (inorganic) and 100 mg/kg (organic). The purpose of this study was to evaluate the cumulative effect of recommended inorganic As in a long term exposure in lactating goats. In rat model, beneficial role of antioxidants have been reported earlier against As toxicity (Ramanathan et al., 2002; Nandi et al., 2006). Beneficial role of feeding ascorbic acid as an antioxidant has been reported in rabbit (Rabbani et al., 2003), rat (Nandi et al., 2006) and in rodents (Rana et al., 2010). Likely, vitamin E is a dietary essential which act as first line of defence in ruminants against pro-oxidant. As the vitamin E requirement of all classes goat is 100 IU/kg (Morand-Fehr, 1981), therefore 100 and 150 IU/kg doses are decided to evaluate the antioxidant effect. Taking into consideration of the above facts, the present investigation was undertaken to assess the protective effect of vitamin E at two levels against As induced changes in hematological and plasma biochemical variables indicative of hepatic and renal functions in lactating goats.
A total of 24 crossbred lactating goats (2nd to 3rd stage of lactation) were selected from the Cattle Yard of National Dairy Research Institute, Karnal, India and distributed randomly into 4 groups of 6 each. The distribution of the animals was done on the basis of average milk yield (1.61±0.04 kg/d) and body weight (36.10±0.11 kg). The experimental feeding period was of 12 months. The details of the treatments are as follows:
The goats were housed in well ventilated pens having facilities for individual feeding. Deworming schedule was followed at the beginning of the experiment and thereafter, at six month interval. The experiment was performed with approval from Institute (NDRI, India) Animal Ethics Committee (IAEC No. 28/09-21/11/2009). Before the trial, animals were adapted to the new environment and fed As and vitamin E capsule for 7 days. The animals were fed as per NRC (1981) feeding standard to meet their nutrient requirements. The concentrate mixture was offered in the morning in the plastic tubs whereas, the chaffed green fodder was offered at 11:00 AM. As it was a long term study, two types of fodder either berseem or maize depending upon the season/availability was provided. Concentrate mixture (maize 33%, groundnut cake (oiled) 21%, mustard oil cake (oiled) 12%, wheat bran 20%, deoiled rice bran 11%, mineral mixture 2% and common salt 1%) having CP 19.81% and TDN 70% was procured from Godrej Agrovet Pvt Ltd. Composition of roughage and concentrate mixture was estimated by drawing weekly samples and every effort was made to maintain the supply of the given fodder of similar composition. Content of As in concentrate mixture, berseem and maize were 1.116, 0.10 and 0.09 mg/kg and content of vitamin E were 10.72, 24.86 and 5.99 mg/kg respectively. 50 mg elemental As/kg dietary DM as sodium arsenite (s-d-Fine-Chem Limited, India, purity 98.5%) and 100 and 150 IU vitamin E/kg dietary DM as dl-α-tocopheryl acetate (Lutavit, BASF, Germany, minimum assay 50%) was weighed, packed in gelatine capsules. Arsenic containing capsules were administered orally to each goat of T1, T2 and T3 groups whereas vitamin E capsules of 100 IU and 150 IU doses were given to each goat of T2 and T3 groups, respectively. The concentration of As was also determined in the water that the animals drank and was found to be 0.003 mg/L. Proximate analysis (DM, CP, EE, CF, NFE and ash) of concentrate mixture, green fodder (berseem and maize) was performed according to methods described in AOAC (2005).
Blood samples were collected aseptically from jugular vein of each animal using heparinized vacutainer tube (Becton Dickinson India Pvt. Ltd., New Delhi, India). Immediately after collection, tubes were placed in freeze (4°C) and analyzed subsequently. Plasma was harvested subsequently by centrifuging the whole blood samples at 840 g for 15 min in a portable centrifuge machine (HERMLE Labortechnik GmbH, Germany). The heparinized plasma samples were stored at −20°C in storage vials and analyzed subsequently.
The haemoglobin (Hb) content of blood was estimated by Drabkin cyanomethemoglobin method (Drabkin, 1944). Blood leukocytes (TLC) and lymphocyte % were done using Neubauer chamber.
Activity of plasma AST and ALT was estimated following the method of Reitman and Frankel (Reitman and Frankel, 1957) using commercial kit (Span Diagnostics Ltd, Surat, India) and the values were expressed as U/L of plasma. Plasma Total protein was measured using modified Biuret method (Koller, 1984) and values were expressed in gm/dl. Plasma creatinine was measured spectrophotometrically using alkaline picrate method (Bonses and Taussky, 1945).
The data were analyzed using two way repeated measures ANOVA (Sigma Plot software, version 11.0; SPSS Inc., Chicago IL, USA) with two factors i.e., different treatment groups and periods. All results were compared to control animals, as well as to the As exposed animals, in order to elucidate the possible protective effect of vitamin E supply on As toxicity. Tukey’s all pair wise multiple comparison tests have been used to compare means between the different treatment groups. The results were represented as mean±SE in the treatment and period interaction table mentioning the significance level. The specific p values were mentioned in the text for where there was significant difference found.
After an initial increase (p = 0.034) in Hb concentration at 2 months in T2, thereafter there was gradual decline in Hb content, thereby suggesting that As administration had an adverse effect as the period of exposure was increased thus confirming its cumulative potential which might have been reflected in depression of Hb (Table 1). Treatment×period interaction (p<0.001) was observed during the study. Both T2 and T3 groups started to show its positive effect in increasing Hb compared to T1 from 8th (p<0.001) and 6th (p = 0.015) months onwards respectively and but statistically there was no difference (p>0.05) between these groups thus it can be concluded that vitamin E supplementation can alleviate adverse effect on Hb due to As supplementation. But the final value in T2 and T3 was different (p<0.001) from control, so dose of vitamin E was not sufficient enough to completely mitigate the adverse effect caused by As. It is also evident from the overall mean values of dietary treatment.
In all As supplemented groups showed a periodically declining trend of TLC compared to control thus indicating its adverse effect (Table 2). Group T1 showed its adverse effect (p = 0.005) from 7 months whereas T2 and T3 were effective (p<0.001) in mitigation of adverse effect from 10 months onwards. As the experimental feeding was continued further, the declining trend maintained and the group T1 showed maximum reduction at the end of 12 months feeding. From the overall mean values of treatments, it was found that vitamin E supplementation at its higher doses significantly mitigate As induced reduction of blood TLC. Treatment×period interaction (p<0.001) was found during the experimental period.
The present study revealed that As exposed goats suffered from hematological abnormality and these findings were similar to those reported in cattle selected from As contaminated zone (Pandey et al., 2005; Rana et al., 2010). Similarly, Biswas et al. (1998) and Pandey et al. (2005) recorded decreased level of Hb and marked leucopenia in experimentally produced As toxicity in goats. Reduction of Hb and TLC might be due to interference in metabolism, suppression of the granulopoietic activity of bone marrow by residual toxicants (Ianchev, 2001; Rana et al., 2008), increased rate of destruction or reduction in rate of formation of erythrocytes and altered cellular composition of blood leading to anemia, anisocytosis, eosinophilia and leucopenia and also due to impaired absorption of folic acid (Hardman and Limbird, 1996) and disruption of liver biosynthesis of heme (Woods and Fowler, 1986). Regarding vitamin E effect, decreasing trend of Hb and TLC was observed in As with vitamin E supplemented animals compared to control but that was not statistically significant. The results were in agreement with findings by Patel et al. (2009) and Vaswani et al. (2010) in growing kids.
Due to experimental feeding AST values were increased at the end of experiment in control, T1, T2 and T3 groups, respectively. Results showed that there was periodical increase in plasma AST activities in all the treatment groups as compared to control which remained almost similar (Table 3). Treatment×period interaction (p<0.001) was observed during the study. In As supplemented group, increase (p = 0.005) in AST activities was found from 4 months onwards. This clearly indicated initiation of adverse effect of As supplementation. Both the vitamin E supplemented groups showed protective effect (p<0.01) of As from 9 months onwards however between the two doses there was no (p>0.05) difference. Though at the end of experiment AST values in T2 and T3 were lower than those in group T1 but still differed (p<0.001) as compared to control. From the overall mean values it was found that vitamin E supplementation at its higher doses ameliorate As induced increase in AST activity.
In T1, T2 and T3 groups, with the increase of duration of exposure there was gradual increase of ALT activities (Table 4). Treatment×period interaction (p = 0.001) was also encountered during the experimental period. T1 groups started showing its adverse effect (p = 0.024) after one month of As supplementation. Up to 8 months activity in T3 was comparable to control. After that in both T2 and T3 groups, activities were increased (p<0.001) in comparison to control group up to the end of experiment but less than T1. From the overall mean values it was observed that vitamin E at both the doses was not able to mitigate significantly As induced increase in ALT activities.
In the present investigation, there was an increased activity of AST and ALT in As supplemented animals. As a toxic metal, arsenic undergoes biotransformation in the liver and cause hepatic as well as systemic oxidative stress (Nandi et al., 2006). However, the liver hepatocytes are affected by the As as it binds with the thiol group of enzymes and protein present in the cells thus it disfuctionate the hepatocytes plasma membrane leading to increased AST and ALT activities (Woods and Fowler, 1986). Similar observations have been reported by Pandey et al. (2005), Vaswani et al. (2010) and Patel et al. (2009) in As supplemented goat. Rana et al. (2010) also reported significant elevation (p<0.05) in plasma ALT (7.452±0.10) and AST (33.21±0.12) activities in cattle of As contaminated area (Ghentugachi village, Nadia district) compared to uncontaminated zone (6.51±0.10 and 28.23± 0.21) that might be resulted from As mediated hepatic injury. However, Mishra et al. (2005) reported non significant effect of dietary supplementation of As at 50 ppm on ALT activity in crossbred calves during 90 days of study. The more pronounced adverse effect of As on serum transaminases activities is of significance in view of the involvement of these variables in heme synthesis and hepatic function. In the present experiment, supplementation of vitamin E was able to reduce activity of these enzymes that might be due to its ability to protect free radicals responsible for oxidative stress and thereby inhibiting the production of ROS. Abbas (2002) also reported the protective effect of vitamin E in reducing AST and ALT activity to a normal level when injection of vitamin E at 20 mg and Se at 0.22 mg/kg body weight was given in Saibi lambs. In conflict with the result, Vaswani et al. (2010) reported non significant (p>0.05) effect of vitamin E supplementation on these enzyme activities during three months of experimental feeding of As. That might be due to lower dose (50 IU/kg DM) of vitamin E.
Overall, there was no effect of dietary treatments on plasma total protein. At the end of the experiment T1 group showed lower (p = 0.048) plasma protein compared to control group. Periodically there was decline trend observed (Figure 1). There was no treatment×period interaction. Vitamin E in both the doses produced significant effect in improving total protein level at the end of experiment. There was increased trend (p<0.05) of plasma creatinine level in all As treated groups. Vitamin E in both the doses produced significant effect (p<0.05) in improving creatinine level at the end of experiment. There was no treatment×period interaction (Figure 2).
Serum biochemistry profiles are sensitive serological indicator of kidney toxicity. Decreased serum total protein in the trial was similar to the results obtained by Nandi et al. (2005) in rats but in contrast to the report of Uthus (2001) in rats, Biswas et al. (2000) in goats and Wang et al. (2006) in pigs. Hypoproteinaemia in arsenic-induced animals is likely to be due to marked destruction and disintegration of parenchymatous tissues. It is also possible that severe nephrotoxic lesions caused drainage of protein through the urine, resulting to hypoproteinaemia. The elevation of serum protein after vitamin E supplementation was probably due to decreased hepatic insulin resistance allowing insulin to stimulate the incorporation of amino acids into protein (Manning et al., 2004). Increased plasma creatinine was similar to Nandi et al. (2005) but in contrast with Wang et al. (2006). Increased creatinine level indicates initiation of renal infliction due to persistent exposure of As. Blood creatinine is a common test used to evaluate kidney function. So, kidney dysfunction (alteration in glomerular filtration) results in decreased creatinine and blood urea nitrogen clearance and therefore increases their blood levels (Kaneko, 1980). Vitamin E supplementation reduced the As induced increase in creatinine level which may be due to the antioxidant effect of the vitamins.
Critical perusal of the results suggested that As exposure resulted in varying degree of changes in hemato-biochemical parameters in lactating goats. Concomitant treatment with vitamins such as α-tocopherol reduced As burden as compared to untreated group but the reduction was not sufficient enough to be comparable to that of unexposed controls, thus revealed specific protection of α-tocopherol from biochemical damage and thus may be helpful to combat As-associated sufferings in animal.
Figure 1
Effect of vitamin E supplementation on plasma total protein (g/dl) level in As fed goats. Bar indicate means±SE. Means followed by the same letter are not significantly different from each other (p<0.05).
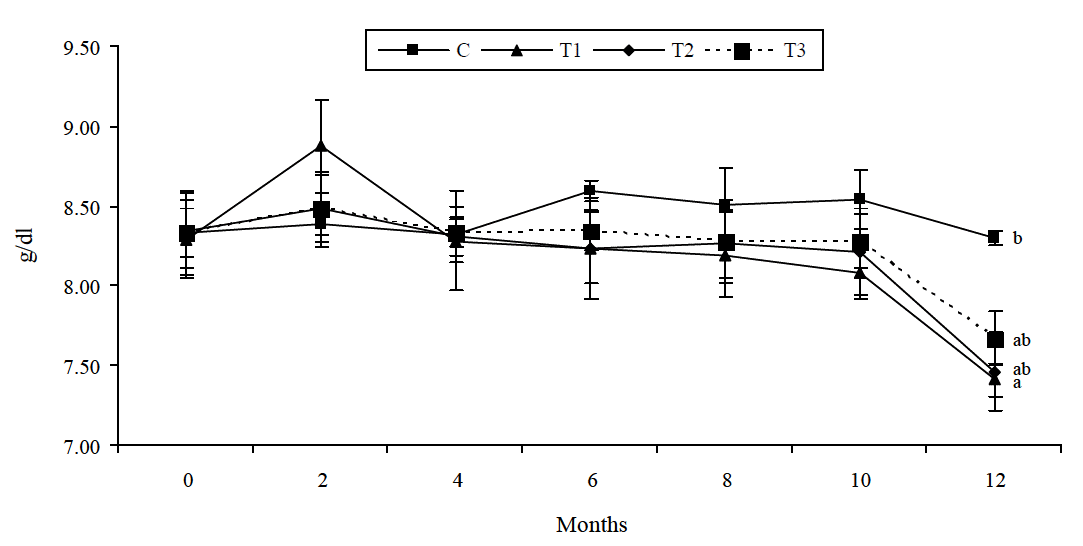
Figure 2
Effect of vitamin E supplementation on plasma creatinine level in As fed goats. Bar indicate means±SE. Means followed by the same letter are not significantly different from each other (p<0.05).

Table 1
Effect of vitamin E supplementation on blood Hb (gm %) in As fed goats
Table 2
Effect of vitamin E supplementation on blood total leukocyte count (×103/mm3) in As fed goats
Table 3
Effect of vitamin E supplementation on AST activity (U/L) in As fed goats
Table 4
Effect of vitamin E supplementation on ALT activity (U/L) in As fed goats
REFERENCES
Abbas SF. 2002. Effect of vitamin E and selenium injections on lamb viability, growth performance and some blood serum constituents in Saibi lambs. Assiut Vet Med J 47:129–136.
AOAC. 1990. Official methods of analysis Association of Official Analytical Chemists. 15th EdWashington, DC, USA:
Biswas U, Sarkar S, Bhowmik MK, Roy S. 1998. Clinicopathological profile of induced chronic arsenic toxicity in goats. Indian J Anim Sci 68:320–323.
Biswas U, Sarkar S, Bhowmik MK, Samanta SK, Biswas S. 2000. Chronic toxicity of arsenic in goats: clinicobiochemical changes, pathomorphology and tissue residues. Small Rumin Res 38:229–235.


Bonses RW, Taussky HH. 1945. On the colorimetric determination of creatinine by the Jaffe reaction. J Biol Chem 158:581–591.

Drabkin DL. 1944. Photometry and spectrometry: Medical physics. 1:Year book medical publishers Inc; Chicago, USA:
Garcia-Shavez E, Jimenez I, Segura B, Razo LMD. 2006. Lipid peroxidative damage and distribution of inorganic and its metabolite in the rat nervous system after arsenite exposure: influence of alpha tocopherol. Neurotoxicology 27:1024–1031.


Hardman G, Limbird LE. 1996. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. 9th ednMcGraw-Hill Companies; New York: p. 633–634.
Healy SM, Aposhian AV. 1999. Diversity of inorganic arsenite transformation. Biol Trace Elem Res 60:249–266.

Ianchev I. 2001. Influence of some geo-chemical ecological factors on some blood hematological characteristics in sheep from Chiprovitei. Zhivotnovu d-Nauki 38:41–43.
Kaneko JJ. 1980. Clinical biochemistry of domestic animals. 3rd EdnAcademic press; NY, USA:
Koller A. 1984. Proteins. Clinical Chemistry: theory, analysis and correlation. Kaplan LA, Pesce AJ, editorsC.V.Mosby; Toranto, Canada: p. 1268–1327.
Liu SX, Athar M, Lippai I, Waldren C, Hei TK. 2001. Induction of oxy-radicals by arsenic: implication for mechanism of genotoxicity. Proc Natl Acad Sci 98:1643–1648.



Manning PJ, Suther WHF, Walker RJ. 2004. Effect of high dose vitamin E on insulin resistance and associated parameters in overweight subjects. Diabetes Care 27:2166


Mishra CS, Mani V, Kaur H. 2005. Effect of arsenic on immunity, oxidative enzyme and various haematological parameters in crossbred calves. Asian-Aust J Anim Sci 18:497–501.

Morand-Fehr P. 1981. Goat Production. Gall G, editorp. 193–232. Academic Press; NY, USA:
Nandi D, Patra RC, Swarup D. 2005. Effect of cysteine, methionine, ascorbic acid and thiamine on arsenic induced oxidative stress and biochemical alterations in rats. Toxicology 211:26–35.


Nandi D, Patra RC, Swarup D. 2006. Oxidative stress indices and plasma biochemical parameters during oral exposure to arsenic in rats. Food Chem Toxicol 44:1579–1584.


NRC. 1981. Nutrient requirements of goats: angora, dairy and meat goats in temperate and tropical countries. National Academy Press; Washington DC, USA:
Pandey PK, Roy M, Roy S. 2005. Clinico-haematological and biochemical alterations in acute arsenic toxicity in goats. Indian J Vet Med 25:57–60.
Patel AK, Kewalramani N, Mani V, Kaur H. 2009. Effect of vitamin E supplementation in growing kids fed on arsenic containing diet. Indian J Anim Nutr 26:9–16.
Rabbani GH, Saha SK, Mastura A, Farzana M. 2003. Antioxidants in detoxification of arsenic induced oxidative injury in rabbits. J Environ Sci Health A 38:273–287.

Ramanathan K, Balakumar BS, Paneerselvam C. 2002. Effects of ascorbic acid and α-tocopherol on arsenic-induced oxidative stress. Hum Exp Toxicol 21:675–680.


Rana T, Bera AK, Das S, Bhattacharya D, Bandyopadhyay S, Pan D, Das SK. 2010. Effect of chronic intake of arsenic-contaminated water on blood oxidative stress indices in cattle in an arsenic-affected zone. Ecotoxicol Environ Saf 73:1327–1332.


Rana T, Sarkar S, Mandal T, Batabyal S. 2008. Hemato-biochemical profiles of affected cattle at arsenic prone zone in Haringhata block of Nadia District of West Bengal in India. Inter J Hematol 4:2
Reitman S, Frankel S. 1957. Colorimetric method for the determination of SGOT and SGPT. Am J Clin Pathol 33:97
Santra A, Das Gupta J, De BK, Roy B, Guha Mazumer DN. 1999. Hepatic manifestations in chronic arsenic toxicity. Indian J Gastroenterol 18:152–155.

Uthus EO. 2001. High dietary arsenic exacerbates copper deprivation in rats. J Trace Elem Exp Med 14:43–55.

Vaswani S, Mani V, Kewalramani N, Kaur H. 2010. Mitigation of adverse effects of arsenic by supplementing vitamin E in crossbred kids maintained at low protein diet. Indian J Anim Nutr 27:347–354.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print





