 |
 |
| Anim Biosci > Volume 36(7); 2023 > Article |
|
Abstract
Objective
Two serine protease inhibitors, peptidase inhibitor 3 (PI3) and secretory leukocyte protease inhibitor (SLPI), play important roles in protease inhibition and antimicrobial activity, but their expression, regulation, and function at the maternalŌĆōfetal interface in pigs are not fully understood. Therefore, we determined the expression and regulation of PI3 and SLPI in the endometrium throughout the estrous cycle and at the maternalŌĆōfetal interface in pigs.
Methods
Endometrial tissues during the estrous cycle and pregnancy, conceptus tissues during early pregnancy, and chorioallantoic tissues during mid to late pregnancy were obtained, and the expression of PI3 and SLPI was analyzed. The effects of the steroid hormones estradiol-17╬▓ (E2) and progesterone (P4) on the expression of PI3 and SLPI were determined in endometrial explant cultures.
Results
PI3 and SLPI were expressed in the endometrium during the estrous cycle and pregnancy, with higher levels during mid to late pregnancy than during the estrous cycle and early pregnancy. Early-stage conceptuses and chorioallantoic tissues during mid to late pregnancy also expressed PI3 and SLPI. PI3 protein and SLPI mRNA were primarily localized to endometrial epithelia. In endometrial explant cultures, the expression of PI3 was induced by increasing doses of P4, and the expression of SLPI was induced by increasing doses of E2 and P4.
The innate immune system is a first-line defense mechanism against infection and inflammation [1]. The innate immune system is composed of physical barriers, including tight junctions and the mucus layer of epithelium; cellular components, including natural killer cells, neutrophils, monocytes, and macrophages; and humoral components, including naturally occurring antibodies, complements, and antimicrobial peptides (AMPs) [2]. Among those innate immune system components, AMPs have inhibitory effects against bacteria, fungi, parasites, and viruses and include a class of small peptides that contains cathelicidins, ╬▒- and ╬▓-defensins, peptidase inhibitor 3 (PI3), secretory leukocyte protease inhibitor (SLPI), and the S100A protein family [3]. Most AMPs are produced by epithelial and inflammatory cells, and AMP deficiency causes vulnerability to infection and inflammation [3]. In addition to their antimicrobial activity, AMPs are involved in modulating inflammation and activating the adaptive immunity [3].
Among the AMPs, PI3, and SLPI are antiproteases that inhibit serine proteases such as cathepsin G, chymotrypsin, and neutrophil elastase [4]. PI3 and SLPI are structurally related in that they have a whey acidic protein domain that is responsible for protease inhibition [5]. Activated neutrophils secrete numerous neutrophil serine proteases, including neutrophil elastase, proteinase 3, and cathepsin G [6]. Because neutrophil serine proteases have the potential to injure host tissues, serine protease inhibitors such as PI3 and SLPI are important for maintaining homeostasis by inhibiting proteolysis [6]. PI3 and SLPI are expressed by neutrophils, macrophages, the decidua, and various epithelial cells in the respiratory, intestinal, and genital tracts [3]. The expression of PI3 and SLPI is upregulated by alarm signals such as bacterial lipopolysaccharide, interleukin (IL)-1╬▓, tumor necrosis factor (TNF)-╬▒, and neutrophil elastase [4,7]. PI3 expression is also induced by neutrophil elastase in alveolar epithelial cells [8].
The innate immune system in the female reproductive tract helps to protect the female from pathogens during the estrous cycle and during establishment and maintenance of pregnancy and parturition [9]. AMPs play an important role in the maintenance of pregnancy because infections are associated with various adverse pregnancy outcomes, including eclampsia, retarded fetal growth, recurrent miscarriage, and premature rupture of membranes [3]. The expression of PI3 and SLPI in female reproductive tissues has been reported in humans and pigs. In humans, SLPI is expressed in the endometrium, decidua, and trophoblast [10]. The expression of SLPI is highest during the late secretory phase in the endometrial epithelium, and SLPI protein is localized to glandular epithelial cells in the endometrium and the decidua [10]. In pigs, SLPI is expressed in the endometrium, with increased level during mid to late pregnancy [11,12]. PI3 expression is highest during menstruation, and PI3 protein is localized to endometrial neutrophils in humans [13]. Progesterone upregulates the expression of SLPI, but not PI3, in human breast epithelial cells [14]. PI3 expression is upregulated by IL-1╬▓ and a combination of IL-1╬▓ and TNF-╬▒ in human primary endometrial epithelial cells [15].
The expression of SLPI in the endometrium throughout the estrous cycle and the expression and regulation of PI3 in the endometrium during the estrous cycle and at the maternalŌĆōconceptus interface during pregnancy have not been fully studied in pigs. Therefore, we determined i) the expression of PI3 and SLPI in the endometrium throughout the estrous cycle and pregnancy, in conceptus tissues during early pregnancy, and in chorioallantoic tissues during mid to late pregnancy; ii) localization of PI3 protein and SLPI mRNA in the endometrium; and iii) the effects of steroid hormones on PI3 and SLPI expression in endometrial tissue explants.
All experimental procedures involving animals were conducted in accordance with the Guide for Care and Use of Research Animals in Teaching and Research and were approved by the Institutional Animal Care and Use Committees of Yonsei University (No. YWC-P120) and the National Institute of Animal Science (No. 2015-137). Sexually mature, crossbred gilts of similar age (6 to 8 months) and weight (100 to 120 kg) were randomly assigned to either cyclic or pregnant status. Gilts assigned to the pregnant status were artificially inseminated with fresh boar semen at the onset of estrus (Day 0) and 12 h later. The reproductive tracts of the gilts were obtained immediately after slaughter on Days 0 (onset of estrous behavior), 3, 6, 9, 12, 15, or 18 of the estrous cycle (21 days of cycle: Days 0 to 3, estrus; Days 3 to 6, metestrus; Days 6 to 15, diestrus; Days 15 to 0, proestrus) or Days 10, 12, 15, 30, 60, 90, or 114 of pregnancy (n = 3 to 6/d/status). Pregnancy was confirmed by the presence of apparently normal spherical to filamentous conceptuses in uterine flushings on Days 10, 12, and 15 and the presence of embryos and placenta on the later days of pregnancy [16]. Uterine flushings were obtained by introducing and recovering 25 mL of phosphate buffered saline (PBS; pH 7.4) into each uterine horn. Chorioallantoic tissues were obtained from Days 30, 60, 90, and 114 of pregnancy (n = 3 to 4/d). Endometrial tissues from prepubertal gilts (n = 8, approximately 6 months of age) that had not undergone an estrous cycle, with no corpus luteum formed, were obtained from a local slaughterhouse. Endometrial tissue, dissected free of myometrium, was collected from the middle portion of each uterine horn, snap-frozen in liquid nitrogen, and stored at ŌłÆ80┬░C prior to RNA extraction. For in situ hybridization and immunohistochemistry, cross-sections of endometrium were fixed in 4% paraformaldehyde in PBS (pH 7.4) for 24 h and then embedded in paraffin as previously described [17].
To determine the effects of the steroid hormones estradiol-17╬▓ (E2) and progesterone (P4) on the expression of PI3 and SLPI mRNA in the endometrium, endometrial explant tissues from the prepubertal gilts were cultured as previously described [17]. The endometrium was dissected from the myometrium and placed into warm phenol redŌĆōfree DulbeccoŌĆÖs modified EagleŌĆÖs medium/F-12 culture medium (DMEM/F-12; Sigma, St. Louis, MO, USA) containing penicillin G (100 IU/mL) and streptomycin (0.1 mg/mL). The endometrium was minced with scalpel blades into small pieces (2 to 3 mm3), and aliquots of 500 mg were placed into T25 flasks with serum-free modified DMEM/F-12 containing 10 ╬╝g/mL insulin (Sigma, USA), 10 ng/mL transferrin (Sigma, USA), and 10 ng/mL hydrocortisone (Sigma, USA). Immediately after mincing, the endometrial explants were cultured in the presence of increasing doses of E2 (0, 5, 50, or 500 pg/mL; Sigma, USA) or P4 (0, 0.3, 3, or 30 ng/mL; Sigma, USA) for 24 h with rocking in an atmosphere of 5% CO2 in air at 37┬░C. The doses were chosen to encompass the full range of physiological levels of E2 and P4 [18]. The explant tissues were then harvested, and total RNA was extracted for use in real-time reverse transcription polymerase chain reaction (RT-PCR) to determine the effects of E2 and P4 on the expression of PI3 and SLPI mRNA.
Total RNA was extracted from endometrial and conceptus tissues using TRIzol reagent (Invitrogen Life Technology, Carlsbad, CA, USA) according to the manufacturerŌĆÖs recommendations. The quantity of RNA was assessed spectrophotometrically, and the integrity of the RNA was validated following electrophoresis in 1% agarose gel. Four micrograms of total RNA from endometrial, conceptus, and chorioallantoic tissues were treated with DNase I (Promega, Madison, WI, USA) and reverse transcribed using SuperScript II reverse transcriptase (Invitrogen, USA) to obtain cDNA. The cDNA templates were then diluted 1:4 with sterile water and amplified by PCR using Taq polymerase (Takara Bio, Shiga, Japan). The final PCR reaction volume of 50 ╬╝L contained 3 ╬╝L of cDNA, 5 ╬╝L of 10├Ś PCR buffer, 4 ╬╝L of dNTP mix (2.5 mM), 1 ╬╝L of each primer (20 ╬╝M), 0.3 ╬╝L of Taq polymerase (Takara Bio, Japan), and 36.7 ╬╝L of ddH2O. The PCR conditions, sequences of primer pairs for PI3 and SLPI, and expected product sizes are listed in Table 1. The PCR products were separated on 2% agarose gels and visualized by ethidium bromide staining. The identity of each amplified PCR product was verified by sequence analysis after cloning into the pCRII vector (Invitrogen, USA).
The expression of PI3 and SLPI mRNA in endometrial and chorioallantoic tissues was analyzed by real-time RT-PCR using an Applied Biosystems StepOnePlus system (Applied Biosystems, Foster City, CA, USA). Power SYBR Green PCR master mix (Applied Biosystems, USA) was used for the PCR reactions. The final reaction volume of 20 ╬╝L contained 2 ╬╝L of cDNA, 10 ╬╝L of 2├Ś master mix, 2 ╬╝L of each primer (2 ╬╝M), and 4 ╬╝L of ddH2O. PCR was performed with an initial incubation at 95┬░C for 10 min, followed by 40 cycles of 15 s at 95┬░C and 30 s at 60┬░C. The sequences of primer pairs are listed in Table 1. The results are reported as expression relative to that detected on Day 0 of the estrous cycle in endometrial tissues, that on Day 30 of pregnancy in chorioallantoic tissues, or that detected in control explant tissues after normalization of the transcript amount to the geometric mean of endogenous ribosomal protein L7 (RPL7), ubiquitin B (UBB), and TATA binding protein (TBP) controls by the 2ŌłÆ╬ö╬öCT method, as previously described [19].
To determine the type(s) of cells expressing PI3 protein in the porcine endometrium, immunohistochemistry was performed. Uterine tissue sections (5 ╬╝m thick) were deparaffinized and rehydrated in an alcohol gradient. Tissue sections were washed with PBS with 0.1% (v/v) Tween-20 (PBST), and endogenous peroxidase activity was blocked with 0.5% (v/v) H2O2 in methanol for 30 min. Tissue sections were then blocked with 10% normal goat serum for 30 min at room temperature. Rabbit polyclonal anti-PI3 antibody (3 ╬╝g/mL; Proteintech, Chicago, IL, USA) was added, and sections were incubated overnight at 4┬░C in a humidified chamber. For each tissue tested, purified normal rabbit immunoglobulin G (IgG) was substituted for the primary antibody as a negative control. Tissue sections were washed intensively with PBST. Biotinylated goat anti-rabbit secondary antibody (1 ╬╝g/mL; Vector Laboratories, Burlingame, CA, USA) was added, and sections were incubated for 1 h at room temperature. Following washes with PBST, a streptavidin peroxidase conjugate (GBI Labs, Bothell, WA, USA) was added to the tissue sections, which were then incubated for 10 min at room temperature. The sections were washed with PBST, and aminoethyl carbazole substrate (Vector Laboratories, USA) was added to the tissue sections, which were then incubated for 20 min at room temperature. The tissue sections were washed in water, counterstained with MayerŌĆÖs hematoxylin, and coverslipped. Images were captured using an Eclipse TE2000-U microscope (Nikon, Seoul, Korea) and processed with Adobe Photoshop CS6 software (Adobe Systems, Seattle, WA, USA).
Nonradioactive in situ hybridization was performed to determine the localization of SLPI mRNA expression in the endometrium, as previously described with some modifications [17]. Tissue sections (5 ╬╝m thick) were rehydrated through successive baths of xylene, 100% ethanol, 95% ethanol, diethylpyrocarbonate (DEPC)-treated water, and DEPC-treated PBS. The sections were boiled in citrate buffer (pH 6.0) for 10 min. After being washed in DEPC-treated PBS, they were digested using 5 ╬╝g/mL Proteinase K (Sigma, USA) in TE (100 mM Tris-HCl, 50 mM ethylenediaminetetraacetic acid, pH 7.5) at 37┬░C. After post-fixation in 4% paraformaldehyde, the tissue sections were incubated twice for 15 min each in PBS containing 0.1% active DEPC and then equilibrated for 15 min in 5├Ś saline sodium citrate (SSC). The sections were prehybridized for 2 h at 68┬░C in hybridization mix (50% formamide, 5├Ś SSC, 500 ╬╝g/mL herring sperm DNA, 250 ╬╝g/mL yeast tRNA). Sense and antisense riboprobes for each gene were generated using partial cDNAs cloned into pCRII vectors by linearizing them with appropriate restriction enzymes and labeling them with digoxigenin (DIG)-UTP using a DIG RNA labeling kit (Roche, Indianapolis, IN, USA). The probes were denatured for 5 min at 80┬░C and added to the hybridization mix. The hybridization reaction was carried out overnight at 68┬░C. The prehybridization and hybridization reactions were performed in a box saturated with a 5├Ś SSC 50% formamide solution to prevent evaporation, and no coverslips were used. After hybridization, the sections were washed for 30 min in 2├Ś SSC at room temperature, 1 h in 2├Ś SSC at 65┬░C, and 1 h in 0.1├Ś SSC at 65┬░C. Probes bound to the section were detected immunologically using sheep anti-DIG Fab fragments covalently coupled to alkaline phosphatase and nitro blue tetrazolium chloride/5-bromo-4-chloro-3-indolyl phosphate (toluidine salt) as a chromogenic substrate, according to the manufacturerŌĆÖs protocol (Roche, USA). The tissue sections were then washed in water and coverslipped. Images were captured using an Eclipse TE2000-U microscope (Nikon, Korea) and processed with Adobe Photoshop CS6 software (Adobe Systems, USA).
Prior to the analysis, all data were tested for normality and homogeneity of variances. When necessary, log and square root transformations were performed. Data from the real-time RT-PCR for PI3 and SLPI expression were subjected to analysis of variance using the general linear models procedures of SAS (Cary, NC, USA). As sources of variation, the model included day, pregnancy status (cyclic or pregnant, Days 12 and 15 post-estrus), and their interactions to evaluate steady-state levels of PI3 and SLPI mRNA. Data from the real-time RT-PCR performed to assess the effects of day of the estrous cycle (Days 0, 3, 6, 9, 12, 15, and 18) and pregnancy (Days 10, 12, 15, 30, 60, 90, and 114) in the endometrium and the effects of day of pregnancy in chorioallantoic tissues (Days 30, 60, 90, and 114) were analyzed using a least squares regression analysis. Data are presented as mean with standard error of the mean. A p-value <0.05 was considered significant, and p-values 0.05 to 0.10 were considered to indicate a trend toward significance.
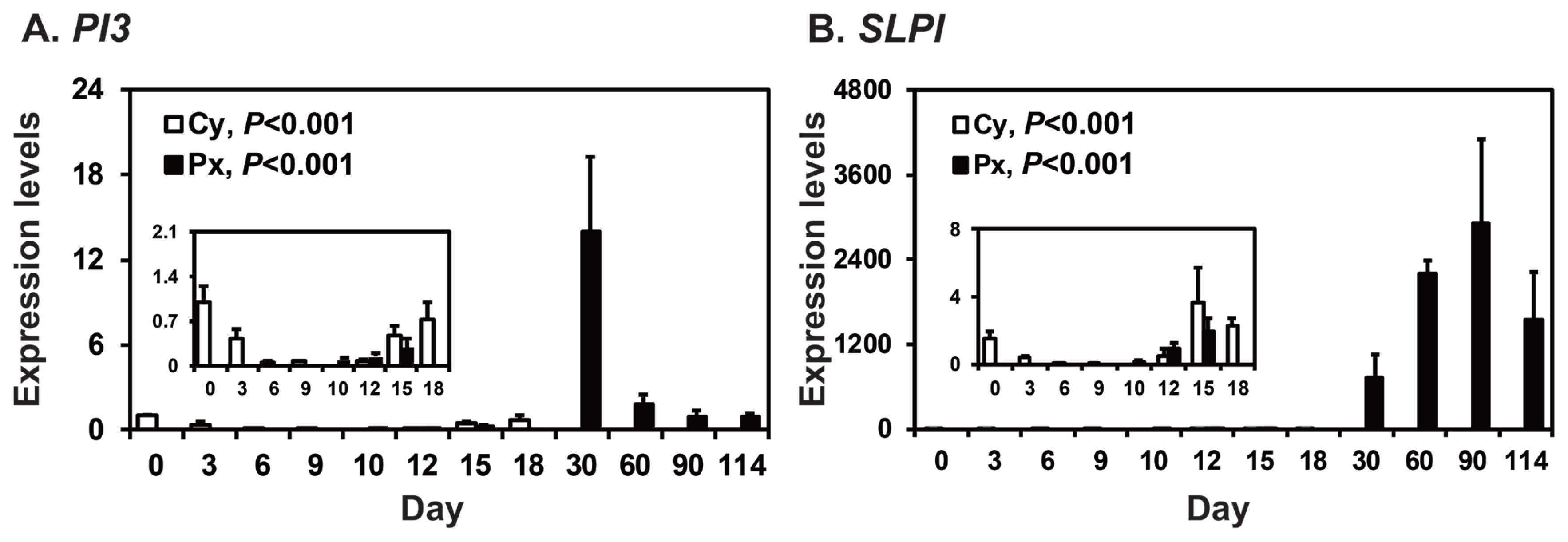
To determine the patterns of PI3 and SLPI expression in the endometrium throughout the estrous cycle and pregnancy in pigs, we measured their relative abundance using real-time RT-PCR (Figure 1). During the estrous cycle, the expression of PI3 and SLPI in the endometrium changed, with the greatest levels in the proestrus phase (quadratic effect of day for PI3 and SLPI, p<0.001). On Days 12 and 15 post-estrus, the expression of PI3 was not affected by day, status, or day├Śstatus interaction, but the expression of SLPI was affected by day (p<0.05), though not by status or day├Śstatus interaction. During pregnancy, the steady-state levels of PI3 and SLPI mRNA in the endometrium changed significantly, with the greatest levels on Day 30 of pregnancy for PI3 mRNA (quadratic effect of day, p<0.001; Figure 1A) and higher levels during mid to late pregnancy than early pregnancy for SLPI mRNA (quadratic effect of day, p<0.001; Figure 1B).
Localization of PI3 protein and SLPI mRNA was determined by immunohistochemistry and in situ hybridization analysis, respectively, in the endometrium during the estrous cycle and pregnancy in pigs. PI3 protein was localized primarily to luminal epithelial (LE) and glandular epithelial (GE) cells in the endometrium on Day 30 of pregnancy and to chorionic epithelial cells of the chorioallantoic membrane during mid to late pregnancy (Figure 2A). Immunostaining signals for PI3 protein were barely detectable in the endometrium during the estrous cycle (data not shown). SLPI mRNA was mainly localized to GE cells during mid to late pregnancy and to LE and GE cells at low levels during early pregnancy (Figure 2B). PI3 protein and SLPI mRNA were detected in the adult small intestine and adult lung, respectively and were used as positive controls.
To determine whether conceptuses expressed PI3 and SLPI mRNA during early pregnancy, we performed RT-PCR using cDNA from conceptuses on Days 12 and 15 of pregnancy. We found that PI3 and SLPI mRNA were expressed in conceptuses on Days 12 and 15 of pregnancy, as well as in the small intestine tissue used as a positive control (Figure 3A). Real-time RT-PCR analysis was performed to determine whether the expression of PI3 and SLPI changed in chorioallantoic tissues during mid to term pregnancy. The expression of PI3 and SLPI mRNA in chorioallantoic tissues changed significantly during mid to term pregnancy, with decreasing and increasing levels, respectively, from Day 30 to term (quadratic effect of day, p<0.05 for PI3 and p<0.01 for SLPI) (Figure 3B).
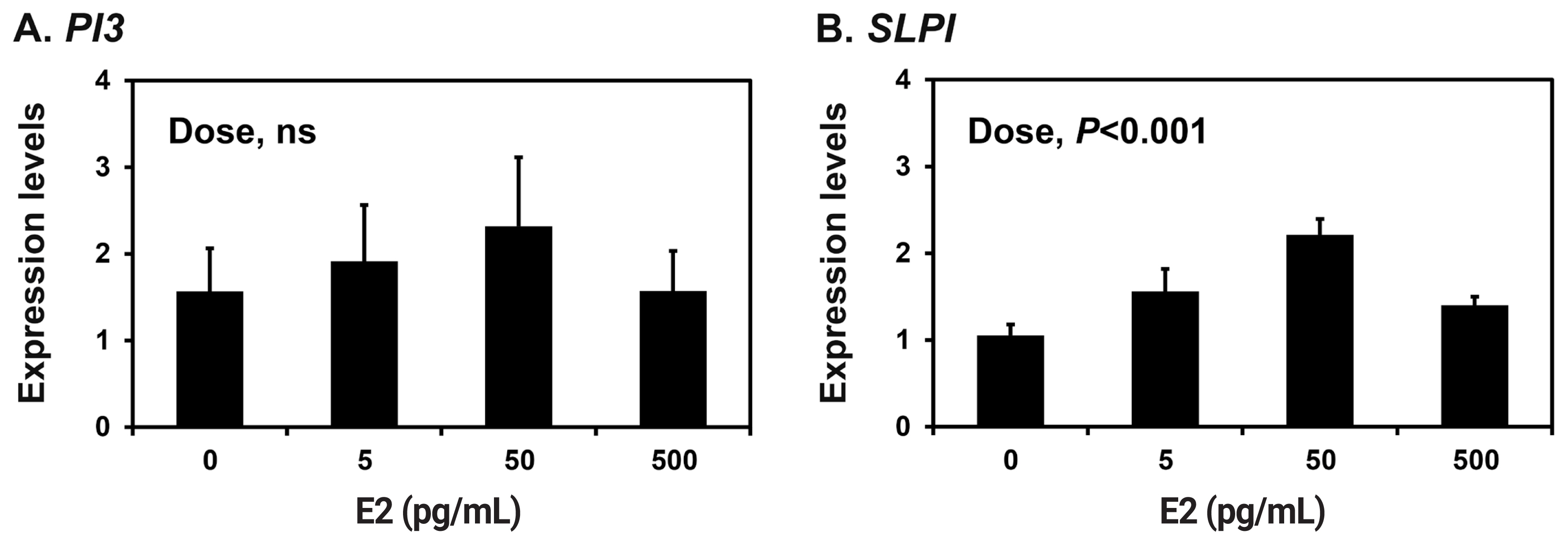
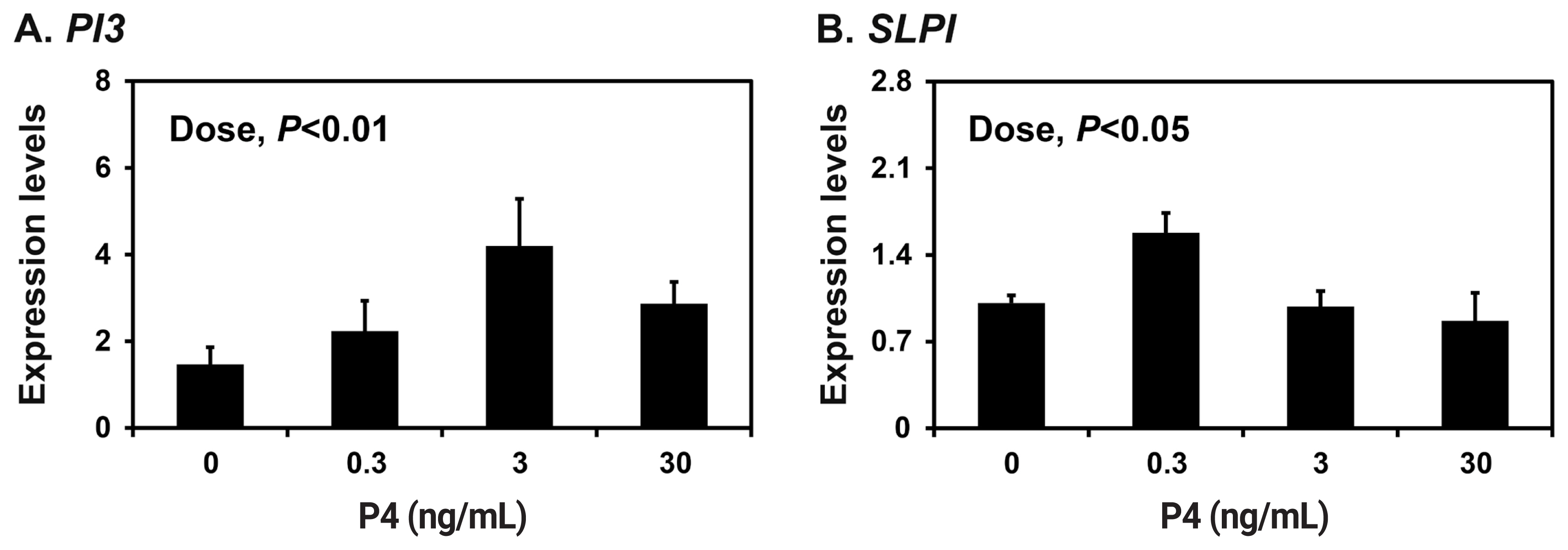
Because the endometrium is a major target of the ovarian steroid hormones E2 and P4 during the estrous cycle and pregnancy [18], we determined the effects of E2 and P4 on the expression of PI3 and SLPI in endometrial explant tissues from prepubertal gilts that had not been exposed to cyclical ovarian hormones. The expression of SLPI (quadratic effect of dose, p<0.001), but not PI3, was upregulated by increasing doses of E2 in endometrial explants (Figure 4A and 4B). The expression of both PI3 and SLPI was upregulated by increasing doses of P4 (quadratic effect of dose for PI3, p<0.05; cubic effect of dose for SLPI, p<0.05) (Figure 5A and 5B).
The novel findings of this study in pigs are as follows: i) PI3 and SLPI are expressed in the endometrium throughout the estrous cycle and pregnancy in an estrous- and pregnancy-stage-dependent manner; ii) PI3 protein is localized to the endometrial epithelia, with a strong signal intensity on Day 30 of pregnancy, and SLPI mRNA is localized to the endometrial epithelia, with a strong signal intensity in GE cells during mid to late pregnancy; iii) early stage conceptuses (Days 12 and 15) and chorioallantoic tissues from Day 30 to term pregnancy express PI3 and SLPI mRNA; and iv) E2 increases the expression of SLPI, and P4 upregulates PI3 and SLPI in endometrial explant tissues.
PI3 and SLPI act as antiproteases and AMPs and are ex pressed at mucosal surfaces in various tissues, such as the lung, small intestine, and endometrium [20]. It is well established that AMPs, including PI3 and SLPI, are expressed in the endometrium during the estrous cycle and pregnancy in various species, including cows, mice, and humans, to protect against potential pathogens [3,13,21]. In humans, the expression of PI3 peaks during menstruation, and the expression of SLPI peaks during the late secretory phase of the menstrual cycle [10,15]. We previously reported that several AMPs, such as the cathelicidin family [22], the S100 family [23,24], and the beta-defensin family (Lee and Ka, unpublished data), are also differentially expressed in the endometrium during the estrous cycle and at the maternalŌĆōconceptus interface during pregnancy in pigs. In this study, we found that PI3 and SLPI are expressed in the endometrium throughout the estrous cycle and pregnancy. Furthermore, the endometrial expression of PI3 and SLPI changed depending on the stage of the estrous cycle and pregnancy; the expression of PI3 and SLPI was highest at the estrus phase and at the proestrus phase, respectively, during the estrous cycle and on Day 30 and during the mid to late stage, respectively, during pregnancy. The expression of SLPI in the endometrium at the conceptus attachment sites during pregnancy was previously reported in pigs [11], but we have further determined the pattern of SLPI expression throughout the estrous cycle and pregnancy. Our results also show that PI3 and SLPI are expressed in conceptus tissues during early pregnancy and in chorioallantoic tissues during mid to late pregnancy. Both PI3 and SLPI are known to have a broad spectrum of activity against Gram-positive and Gram-negative bacteria [3]. Because the stage of increased expression of PI3 and SLPI during the estrous cycle corresponds with the estrogen-dominant period, we postulate that the increased PI3 and SLPI at this stage of the cycle are involved in protecting the endometrium from possible bacterial contamination at mating. In addition, the levels of both PI3 and SLPI in the endometrium were much higher during pregnancy than in the estrous cycle, and PI3 and SLPI were expressed in conceptus and chorioallantoic tissues during pregnancy, which suggests that the antimicrobial activity of PI3 and SLPI could play an important role in protecting the maternalŌĆōconceptus interface from microbial contamination to protect and maintain pregnancy.
The results of this study show that PI3 protein was localized to epithelial cells in both the endometrium and chorioallantoic tissues at the attachment sites, with strong signal intensity on Day 30 of pregnancy. It has already been shown that various AMPs, including PI3 and SLPI, are present in the epithelia of the endometrium and fetal membranes in other species, such as humans and cows [25], suggesting that AMP expression at the maternalŌĆōconceptus interface is common across many mammalian species. As reported for SLPI protein localization at the maternalŌĆōconceptus interface in pigs [26], the expression of SLPI mRNA was primarily localized to endometrial GE cells during mid to late pregnancy and to LE and GE cells at low levels during early pregnancy, suggesting the possibility of SLPI secretion into the maternalŌĆōconceptus interface to protect the conceptus attachment sites during pregnancy.
The endometrium is a major target of estrogen and pro gesterone during the estrous cycle and pregnancy [18], and the expression of PI3 and SLPI changed during the estrous cycle and pregnancy in this study. Although the expression of PI3 under the influence of steroid hormones is not well studied, the expression of SLPI is increased by estrogen in uterine epithelial cells and by progesterone in mammary epithelial cells in humans [27]. Therefore, we postulated that E2 and P4 might regulate endometrial PI3 and SLPI expression. Indeed, the expression of PI3 was upregulated by P4, and SLPI expression was dose-dependently increased by both E2 and P4 in endometrial explants. Our results also coincide with a previous report showing that estrogen and progesterone upregulate the expression of SLPI in porcine endometrial GE cells [28]. Because the expression of SLPI was highest during the estrus phases of the estrous cycle, when the plasma concentration of E2 is at its highest level [18], and during mid to late pregnancy, when chorioallantoic placentas produce both estrogen and progesterone in pigs [29], it is possible that ovarian estrogen is responsible for endometrial SLPI expression during the estrous cycle, and that estrogen and progesterone are together responsible for SLPI expression during mid to late pregnancy in pigs. Although PI3 expression was highest at the estrus phase of the estrous cycle, E2 did not affect its expression, though P4 increased PI3 expression in this study. In human endometrial epithelial cells, pro-inflammatory cytokines such as IL-1╬▓ and TNF-╬▒ are known to increase the expression of PI3 [15]. In pigs, the pro-inflammatory cytokines IL-1╬▓, IL-6, and TNF-╬▒ are expressed in the endometrium during the estrous cycle and pregnancy [30]. Thus, these data suggest that other factors, including pro-inflammatory cytokines, could be involved in the expression of PI3 during the estrogen-dominant period of the estrous cycle.
Trophoblasts are a rich source of proteases that induce degradation of the endometrial epithelium for trophoblast invasion. However, trophoblast invasion is controlled by protease inhibitors, and the degree of invasion determines the criteria for classifying placental types [31]. Pigs form a true epitheliochorial placenta, in which trophoblast invasion does not occur, so the endometrial epithelial cell layer remains intact until term during pregnancy [32]. Although PI3 and SLPI are well known AMPs found in various epithelial tissues that act in the innate immune response, they also act as protease inhibitors, blocking the activity of many enzymes, including cathepsin G, chymotrypsin, and neutrophil elastase [4]. It has been proposed that SLPI is expressed in the endometrium at high levels during late pregnancy in domestic animals that form epitheliochorial placentas, such as pigs, mares, and cows [33]. Thus, it is possible that the PI3 and SLPI mRNA expressed at the maternalŌĆōconceptus interface not only protect the mother and fetus from infections through their antimicrobial activity, but also inhibit trophoblast invasion to enable the development and maintenance of the epitheliochorial placenta through their antiprotease activity.
In conclusion, the results of this study in pigs demonstrate that the serine protease inhibitors PI3 and SLPI are differentially expressed in the endometrium during the estrous cycle and pregnancy and at the maternalŌĆōconceptus interface during pregnancy; PI3 and SLPI expression was localized primarily to endometrial epithelial cells; and steroid hormones induced the expression of PI3 and SLPI in endometrial tissues. Although the detailed functions of PI3 and SLPI need to be further determined, our findings indicate that the antimicrobial proteins PI3 and SLPI are expressed stage-specifically at the maternalŌĆōconceptus interface and might thus play important roles in both protecting maternal and conceptus tissues from potential pathogens and establishing the epitheliochorial placenta by regulating trophoblast invasion.
Notes
Figure┬Ā1
Expression of peptidase inhibitor 3 (PI3) and secretory leukocyte protease inhibitor (SLPI) in the endometrium during the estrous cycle and pregnancy. Endometrial tissue samples from cyclic (Cy) and pregnant gilts (Px) were analyzed by real-time reverse transcription polymerase chain reaction, and data are reported as expression relative to that detected on Day 0 of the estrous cycle after normalization of the transcript amount to the endogenous ribosomal protein L7, TATA binding protein, and ubiquitin B controls. Data are presented as means with standard errors.
Figure┬Ā2
Localization of peptidase inhibitor 3 (PI3) and secretory leukocyte protease inhibitor (SLPI) mRNA in the endometrium during the estrous cycle and pregnancy. (A) Immunohistochemistry for the PI3 protein. Representative images from Days 15 and 30 of pregnancy are shown. A uterine section from Day 30 of pregnancy immunostained with normal immunoglobulin G (IgG) is shown as a negative control, and a tissue section from the small intestine is shown as a positive control. (B) In situ hybridization for SLPI mRNA. Representative images during the estrous cycle and pregnancy are shown. A uterine section from Day 30 of pregnancy hybridized with a digoxigenin-labeled sense SLPI cDNA probe (Sense) is shown as a negative control, and a tissue section from the lung is shown as a positive control. D, day; C, estrous cycle; P, pregnancy; LE, luminal epithelium; GE, glandular epithelium; CE, chorionic epithelium; St, stroma; BV, blood vessel. Bars = 100 ╬╝m, 20 ╬╝m in inset.

Figure┬Ā3
Expression of peptidase inhibitor 3 (PI3) and secretory leukocyte protease inhibitor (SLPI) in conceptuses from Days 12 and 15 of pregnancy and in chorioallantoic tissues during late pregnancy. (A) RT-PCR analysis of PI3 and SLPI mRNA in conceptuses on Days 12 and 15 of pregnancy. Ribosomal protein L7 (RPL7) was used as a positive control in the polymerase chain reaction (PCR) analysis, and small intestine was used as a positive control. RTase +/ŌłÆ, with (+) or without (ŌłÆ) reverse transcriptase; M, molecular marker; D12 Endo, endometrium on Day 12 of pregnancy; D12 Con, Day 12 conceptus; D15 Con, Day 15 conceptus. (B) Real-time reverse transcription PCR analysis of the expression of PI3 and SLPI mRNA in chorioallantoic tissues on Days 30, 60, 90, and 114 of pregnancy. Data are reported as expression relative to that detected on Day 30 of pregnancy after normalization of the transcript amount to the endogenous RPL7, TATA binding protein, and ubiquitin B controls, and data are presented as means with standard errors.

Figure┬Ā4
Effects of estradiol-17╬▓ (E2) on the expression of peptidase inhibitor 3 (PI3) and secretory leukocyte protease inhibitor (SLPI) in endometrial explant cultures. Endometrial explants from prepubertal gilts were cultured in the presence of increasing doses of E2 (0, 5, 50, or 500 pg/mL) at 37┬░C for 24 h. The abundance of mRNA expression shown in the real-time reverse transcription polymerase chain reaction analyses is relative to that of PI3 and SLPI mRNA in the control group of endometrial explants (0 ng/mL) after normalization of the transcript amounts to ribosomal protein L7, TATA binding protein and ubiquitin B mRNA. Data are presented as least squares means with standard errors. For each treatment, experiments were performed in triplicate with endometria from eight gilts.
Figure┬Ā5
Effects of progesterone (P4) on the expression of peptidase inhibitor 3 (PI3) and secretory leukocyte protease inhibitor (SLPI) in endometrial explant cultures. Endometrial explants from prepubertal gilts were cultured in the presence of increasing doses of P4 (0, 0.3, 3, or 30 ng/mL) at 37┬░C for 24 h. The abundance of mRNA expression shown in the real-time reverse transcription polymerase chain reaction analyses is relative to that for PI3 and SLPI mRNA in the control group of endometrial explants (0 ng/mL) after normalization of the transcript amounts to ribosomal protein L7, TATA binding protein, and ubiquitin B mRNA. Data are presented as least squares means with standard errors. For each treatment, experiments were performed in triplicate with endometria from eight gilts.
Table┬Ā1
Summary of primer sequences for real-time RT-PCR and RT-PCR and expected product sizes
REFERENCES
1. Chaplin DD. 1. Overview of the human immune response. J Allergy Clin Immunol 2006; 117:S430ŌĆō5. https://doi.org/10.1016/j.jaci.2005.09.034


2. Gasteiger G, DŌĆÖOsualdo A, Schubert DA, Weber A, Bruscia EM, Hartl D. Cellular innate immunity: an old game with new players. J Innate Immun 2017; 9:111ŌĆō25. https://doi.org/10.1159/000453397


3. Frew L, Stock SJ. Antimicrobial peptides and pregnancy. Reproduction 2011; 141:725ŌĆō35. https://doi.org/10.1530/REP-10-0537


4. Sallenave JM. Secretory leukocyte protease inhibitor and elafin/trappin-2: versatile mucosal antimicrobials and regulators of immunity. Am J Respir Cell Mol Biol 2010; 42:635ŌĆō43. https://doi.org/10.1165/rcmb.2010-0095RT


5. Moreau T, Baranger K, Dade S, Dallet-Choisy S, Guyot N, Zani M. Multifaceted roles of human elafin and secretory leukocyte proteinase inhibitor (SLPI), two serine protease inhibitors of the chelonianin family. Biochimie 2008; 90:284ŌĆō95. https://doi.org/10.1016/j.biochi.2007.09.007


6. Pham CT. Neutrophil serine proteases: specific regulators of inflammation. Nat Rev Immunol 2006; 6:541ŌĆō50. https://doi.org/10.1038/nri1841


7. Pfundt R, Wingens M, Bergers M, Zweers M, Frenken M, Schalkwijk J. TNF-alpha and serum induce SKALP/elafin gene expression in human keratinocytes by a p38 MAP kinase-dependent pathway. Arch Dermatol Res 2000; 292:180ŌĆō7. https://doi.org/10.1007/s004030050475


8. Reid PT, Marsden ME, Cunningham GA, Haslett C, Sallenave JM. Human neutrophil elastase regulates the expression and secretion of elafin (elastase-specific inhibitor) in type II alveolar epithelial cells. FEBS Lett 1999; 457:33ŌĆō7. https://doi.org/10.1016/s0014-5793(99)01004-2


9. Amjadi F, Salehi E, Mehdizadeh M, Aflatoonian R. Role of the innate immunity in female reproductive tract. Adv Biomed Res 2014; 3:1https://doi.org/10.4103/2277-9175.124626



10. King AE, Critchley HO, Kelly RW. Presence of secretory leukocyte protease inhibitor in human endometrium and first trimester decidua suggests an antibacterial protective role. Mol Hum Reprod 2000; 6:191ŌĆō6. https://doi.org/10.1093/molehr/6.2.191


11. Reed KL, Blaeser LL, Dantzer V, Green ML, Simmen RCM. Control of secretory leukocyte protease inhibitor gene expression in the porcine periimplantation endometrium: a case of maternal-embryo communication. Biol Reprod 1998; 58:448ŌĆō57. https://doi.org/10.1095/biolreprod58.2.448


12. Farmer SJ, Fliss AE, Simmen RC. Complementary DNA cloning and regulation of expression of the messenger RNA encoding a pregnancy-associated porcine uterine protein related to human antileukoproteinase. Mol Endocrinol 1990; 4:1095ŌĆō104. https://doi.org/10.1210/mend-4-8-1095


13. King AE, Critchley HO, Kelly RW. Innate immune defences in the human endometrium. Reprod Biol Endocrinol 2003; 1:116https://doi.org/10.1186/1477-7827-1-116



14. King AE, Morgan K, Sallenave JM, Kelly RW. Differential regulation of secretory leukocyte protease inhibitor and elafin by progesterone. Biochem Biophys Res Commun 2003; 310:594ŌĆō9. https://doi.org/10.1016/j.bbrc.2003.08.151


15. King AE, Critchley HO, Sallenave JM, Kelly RW. Elafin in human endometrium: an antiprotease and antimicrobial molecule expressed during menstruation. J Clin Endocrinol Metab 2003; 88:4426ŌĆō31. https://doi.org/10.1210/jc.2003-030239


16. Oestrup O, Hall V, Petkov SG, Wolf XA, Hyldig S, Hyttel P. From zygote to implantation: morphological and molecular dynamics during embryo development in the pig. Reprod Domest Anim 2009; 44:Suppl 339ŌĆō49. https://doi.org/10.1111/j.1439-0531.2009.01482.x


17. Yoo I, Jung W, Lee S, Cheon Y, Ka H. Inhibitors of apoptosis: expression and regulation in the endometrium during the estrous cycle and at the maternal-conceptus interface during pregnancy in pigs. Anim Biosci 2022; 35:533ŌĆō43. https://doi.org/10.5713/ab.21.0307


18. Ka H, Seo H, Choi Y, Yoo I, Han J. Endometrial response to conceptus-derived estrogen and interleukin-1beta at the time of implantation in pigs. J Anim Sci Biotechnol 2018; 9:44https://doi.org/10.1186/s40104-018-0259-8



19. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(ŌłÆDelta Delta C(T)) method. Methods 2001; 25:402ŌĆō8. https://doi.org/10.1006/meth.2001.1262


20. Williams SE, Brown TI, Roghanian A, Sallenave JM. SLPI and elafin: one glove, many fingers. Clin Sci (Lond) 2006; 110:21ŌĆō35. https://doi.org/10.1042/CS20050115


21. Ibrahim M, Peter S, Gartner MA, et al. Increased mRNA expression of selected antimicrobial peptides around ovulation and during inflammatory processes in the bovine endometrium postpartum. Theriogenology 2016; 86:2040ŌĆō53. https://doi.org/10.1016/j.theriogenology.2016.06.022


22. Lee S, Yoo I, Han J, Ka H. Antimicrobial peptides cathelicidin, PMAP23, and PMAP37: Expression in the endometrium throughout the estrous cycle and at the maternal-conceptus interface during pregnancy and regulation by steroid hormones and calcitriol in pigs. Theriogenology 2021; 160:1ŌĆō9. https://doi.org/10.1016/j.theriogenology.2020.10.034


23. Lee S, Jang H, Yoo I, Han J, Jung W, Ka H. Unique epithelial expression of S100A calcium binding protein A7A in the endometrium at conceptus implantation in pigs. Asian-Australas J Anim Sci 2019; 32:1355ŌĆō62. https://doi.org/10.5713/ajas.18.0920



24. Jang H, Lee S, Yoo I, et al. Calcium-binding proteins S100A8, S100A9, and S100A12: expression and regulation at the maternal-conceptus interface in pigs. Biol Reprod 2022; 106:1098ŌĆō111. https://doi.org/10.1093/biolre/ioac039


25. Ramuta TZ, Sket T, Starcic Erjavec M, Kreft ME. Antimicrobial activity of human fetal membranes: from biological function to clinical use. Front Bioeng Biotechnol 2021; 9:691522https://doi.org/10.3389/fbioe.2021.691522



26. Simmen RC, Michel FJ, Fliss AE, Smith LC, Fliss MF. Ontogeny, immunocytochemical localization, and biochemical properties of the pregnancy-associated uterine elastase/cathepsin-G protease inhibitor, antileukoproteinase (ALP): monospecific antibodies to a synthetic peptide recognize native ALP. Endocrinology 1992; 130:1957ŌĆō65. https://doi.org/10.1210/endo.130.4.1547723


27. Fahey JV, Wright JA, Shen L, et al. Estradiol selectively regulates innate immune function by polarized human uterine epithelial cells in culture. Mucosal Immunol 2008; 1:317ŌĆō25. https://doi.org/10.1038/mi.2008.20



28. Reed KL, Badinga L, Davis DL, Chung TE, Simmen RCM. Porcine endometrial glandular epithelial cells in vitro: transcriptional activities of the pregnancy-associated genes encoding antileukoproteinase and uteroferrin. Biol Reprod 1996; 55:469ŌĆō77. https://doi.org/10.1095/biolreprod55.2.469


29. Bazer FW, Johnson GA. Pig blastocyst-uterine interactions. Differentiation 2014; 87:52ŌĆō65. https://doi.org/10.1016/j.diff.2013.11.005


30. Yoo I, Kim M, Han J, et al. Pro-inflammatory cytokines and their receptors: expression and regulation in the uterine endometrium during the estrous cycle in pigs. J Embryo Trans 2016; 31:323ŌĆō33. https://doi.org/10.12750/JET.2016.31.4.323

31. Lala PK, Graham CH. Mechanisms of trophoblast invasiveness and their control: the role of proteases and protease inhibitors. Cancer Metastasis Rev 1990; 9:369ŌĆō79. https://doi.org/10.1007/BF00049525


32. Dantzer V. Electron microscopy of the initial stages of placentation in the pig. Anat Embryol (Berl) 1985; 172:281ŌĆō93. https://doi.org/10.1007/BF00318976


33. Badinga L, Michel FJ, Fields MJ, Sharp DC, Simmen RCM. Pregnancy-associated endometrial expression of antileukoproteinase gene is correlated with epitheliochorial placentation. Mol Reprod Dev 1994; 38:357ŌĆō63. https://doi.org/10.1002/mrd.1080380402








 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print





