 |
 |
| Anim Biosci > Volume 36(10); 2023 > Article |
|
Abstract
Objective
Sous-vide cooking offers several advantages for poultry meat, including enhanced tenderness, reduced cooking loss, and improved product yield. However, in duck meat, there are challenges associated with using the sous-vide method. The prolonged cooking time at low temperatures can lead to unstable microbial and oxidative stabilities. Thus, we aimed to assess how varying sous-vide cooking temperatures and durations affect the physicochemical and microbial characteristics of duck breast meat, with the goal of identifying an optimal cooking condition.
Methods
Duck breast meat (Anas platyrhynchos) aged 42 days and with an average weight of 1,400±50 g, underwent cooking under various conditions (ranging from 50°C to 80°C) for either 60 or 180 min. Then, physicochemical, microbial, and microstructural properties of the cooked duck breast meat were assessed.
Results
Different cooking conditions affected the quality attributes of the meat. The cooking loss, lightness, yellowness, Hue angle, whiteness, and thiobarbituric acid reactive substance (TBARS) values of the duck breast meat increased with the increase in cooking temperature and time. In contrast, the redness and chroma values decreased with the increase in cooking temperature and time. Cooking of samples higher than 60°C increased the volatile basic nitrogen contents and TBARS. Microbial analysis revealed the presence of Escherichia coli and Coliform only in the samples cooked at 50°C and raw meat. Cooking at lower temperature and shorter time increased the tenderness of the meat. Microstructure analysis showed that the contraction of myofibrils and meat density increased upon increasing the cooking temperature and time.
Meats require heat treatment to improve palatability and digestive efficiency, and especially to delay microbial growth. Meat proteins undergo modifications depending on the cooking temperature and time, which affect the quality attributes of the meat product, including cooking loss, color, and texture [1]. Meat products can be cooked using various methods of heat transfer, such as convection, conduction, and radiation [2]. Sous-vide is a popular cooking method in which heat is transferred from the water to vacuum-sealed food through conduction [3]. In this cooking method, the vacuum-packed meat is placed in a plastic bag and cooked under controlled temperature for a specific time in a water bath [3]. The meat is then immediately cooled in an ice bath to bypass the optimal growth temperature of microbes [4]. The advantages of sous-vide include uniform heating of the meat, decreased risk of microbial recontamination after heating and during storage—as the meat is vacuum-packaged—increased meat tenderness as the meat is subjected to low heat treatment (50°C to 65°C) for a prolonged period. Moreover, cooking at low temperatures for a long time has economic benefits as it can reduce cooking loss and increase the meat product yield. Several studies have demonstrated that the sous-vide cooking method improves the overall meat quality indices, such as cooking loss, tenderness, and texture [5,6].
Duck meat, known for its unique sensory properties such as color and flavor, is produced and consumed globally, particularly in Asia. Duck meat is classified as red meat due to its higher red muscle fiber content compared to chicken or turkey meat [7]. Additionally, compared to other meats, duck meat has higher levels of unsaturated fatty acids, such as linoleic acid, linolenic acid, and oleic acid [8]. Some studies have investigated the positive health effects of these fatty acid profiles, including anti-obesity effects through the promotion of lipid metabolism in viscera tissues, and hepatic health effects by inhibiting lipotoxicity in HepG2 cells [9,10]. Thus, nutrient-rich duck meat can be a good dietary choice.
Although the sous-vide method has the aforementioned benefit in meat processing, there is limited scientific information that specifically applies to duck meat. Duck meat, when cooked using the sous-vide method which involves cooking at low temperatures for a prolonged period, is susceptible to lipid oxidation and microbial contamination due to its high content of unsaturated fatty acids. Therefore, it may be beneficial to explore optimal sous-vide cooking methods for duck meat. In this study, we investigated the effects of different cooking temperatures (50°C, 60°C, 70°C, and 80°C) and time (60 and 180 min) on the physicochemical and microbial properties of sous-vide cooked duck breast meat.
Pekin ducks (Anas platyrhynchos) aged 42 days with a bodyweight of 1,400±50 g were slaughtered at a local abattoir. The skin, fat and connective tissues were removed from the breast muscles. Next, the breast muscles were vacuum-packaged in an 80 μm polyethylene vacuum pouch, stored at 4°C, and analyzed within two days. The samples were cooked at 50°C, 60°C, 70°C, or 80°C with cooking time of 60 and 180 min for each temperature. Vacuum-packed duck breasts were heated using a circulating thermostatic water bath (VS-1205SW1-0; Vision Science, Daegu, Korea). All samples were chilled in an ice bath after heating to pass the optimum temperature for microbial growth. Cooked duck breast sample stored at 4°C until further analysis.
Pre-weighted duck breast meat was vacuum-packed before cooking. Exudative liquid from cooked duck breast samples was removed, and the samples were chilled until approximately 16°C. Chilled and dried samples were weighted 10 times per treatment. Cooking loss was calculated by comparing the meat weight before and after sous-vide cooking, as follows:
where W0 is the weight of raw meat batter (g), and W1 is weight of cooked meat batter (g).
The color of the duck breast meat was measured on the cutting side of the cooked duck breast using a colorimeter (Minolta Chroma Meter CR-210; Minolta, Tokyo, Japan; Illuminate C, calibrated with a white plate; Y, 93.5; x, 0.3134; y, 0.3197). The color wass described in terms of L* (lightness), a* (redness), b* (yellowness), ΔE (color difference), C* (chroma), H° (hue angle), and whiteness. The ΔE, C*, H°, and whiteness values were calculated using the following equations:
Lipid oxidation was evaluated using the 2-thiobarbituric acid (TBA) method, following the protocols of a previous study with minor modifications [11]. The meat sample (10 g) was homogenized with 50 mL of distilled water at 10,000 rpm for 2 min using a homogenizer (Model AM-7; Nihonseiki Kaisha Ltd., Osaka, Japan). The homogenizer cup and blade were washed with 47.5 mL of distilled water, and the total mixture was transferred to a distillation tube. Next, 2.5 mL of 4 N HCl and 1 mL of antifoam agent (KMK-73; Shin-Etsu Silicone Co., LTD., Seoul, Korea) were added to the mixture in the distillation tube. The mixture was subjected to distillation and 40 mL of the distillate was collected. An aliquot of the distillate (5 mL) was added to a test tube containing 5 mL of 0.02 M TBA in 90% acetic acid (TBA reagent) and mixed using a vortex mixer (Vortex-Genie 2, Bohemia, NY, USA). The tube was sealed with a cap and heated in a water bath at 95°C for 35 min. The samples were then chilled in an ice bath for 10 min. The absorbance of the sample was measured at 538 nm using a UV/VIS spectrophotometer (Optizen 2120 UV Plus; Mecasys Co., Ltd., Daejeon, Korea). The TBA values were expressed as malonaldehyde (MDA) equivalent (mg MDA/kg of sample).
Protein deterioration in the duck breast meat sample was assessed using the volatile basic nitrogen (VBN) method. The meat sample (5 g) in a conical tube (SPL Life Sciences Co., Ltd., Gyeonggi, Korea) was homogenized with 15 mL distilled water at 10,000 rpm for 2 min using a homogenizer (HG-15A; DAIHAN, Wonju, Korea). Next, 30 mL of distilled water was added to the homogenate and mixed using a vortex mixer. The mixture was filtered through a Whatman filter paper No. 1 (Whatman International, Maidstone, UK). The filtrate (1 mL) and 50% K2CO3 were added to the outer section of the Conway micro diffusion cell, while 1 mL of 0.01 N H3BO3 solution and 100 μL of indicator (0.066% methyl red and 0.066% bromocresol green in ethanol) were added to the inner section. The samples were incubated at 37°C for 2 h. Finally, the solution in the inner section was titrated against 0.02 N H2SO4. The VBN content was calculated using the following equation:
where S is the sample weight (mg), a is the volume (mL) of H2SO4 added to the solution obtained from the inner section, b is the volume (mL) of H2SO4 added to the blank, and f is the standard factor of H2SO4.
The tenderness of duck breast meat was measured based on Warner-Bratzler shear force (WBSF) at room temperature using a texture analyzer (TA-XT2i, Stable Micro Systems Ltd., Godalming, UK). The core of the cooked duck breast was obtained using a hand-held coring device (1.3 cm diameter) along with the muscle fiber. A Warner-Bratzler blade with a triangular cut-out notch was used to measure the shear force. The settings of the texture analyzer were as follows: pre-test speed, 2.0 mm/s; post-test speed, 5.0 mm/s; maximum load, 2.0 kg; head speed, 2.0 mm/s; distance, 8.0 mm; force, 5.0 g. The texture analyzer was calibrated to 5 kg before use.
Texture profile analysis (TPA) was performed using a texture analyzer (TA-XT2i; Stable Micro Systems Ltd., England) equipped with a 45° conical probe at room temperature. The central part of the cooked duck breast was cut into a cube with a dimension of 2×2×2 cm. The settings of the texture analyzer were as follows: pre-test speed, 2.0 mm/s; post-test speed, 5.0 mm/s; maximum load, 2.0 kg; head speed, 2.0 mm/s; distance, 8.0 mm; force, 5.0 g. The texture analyzer was calibrated with 5 kg before use.
Microbiological analysis was conducted after the samples were cooked and chilled. The duck breast sample (25 g) was transferred to a sterilized filter bag for homogenization (ELMEX PYXON-20, Tokyo, Japan) and diluted 10-fold using sterile phosphate-buffered saline. The mixture was homogenized using a stomacher (Wisemix WES-400; DAIHAN Scientific, Korea) at level 10 for 1 min. The total viable count (TVC) and psychrophilic bacteria were counted on plate count agar (Merck, Darmstadt, Germany) and standard plate count agar (Oxoid Ltd., Hampshire, UK), respectively. Each agar was then incubated at 37°C for 48 h and at 25°C for 72 h. Escherichia coli (E. coli), coliforms, and lactic acid bacteria (LAB), were counted using Petrifilm (3M, St. Paul, MN, USA). TVC, E. coli, coliforms, and LAB were incubated at 37°C for 48 h. The microbial colonies (30 to 300) were counted and expressed as log10 colony forming unit (CFU)/g sample.
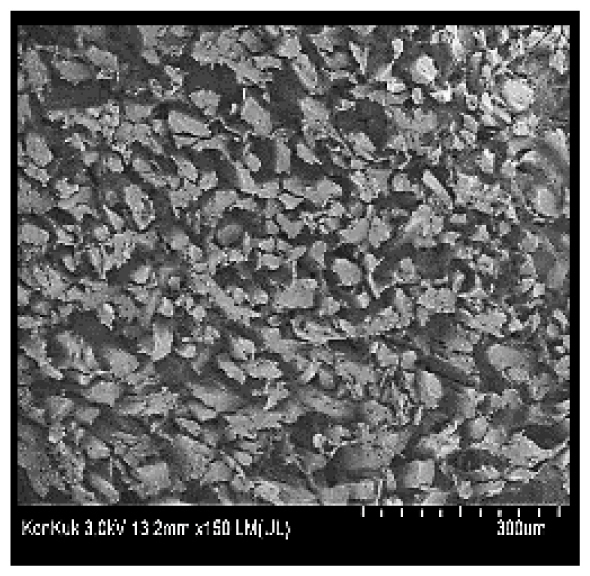
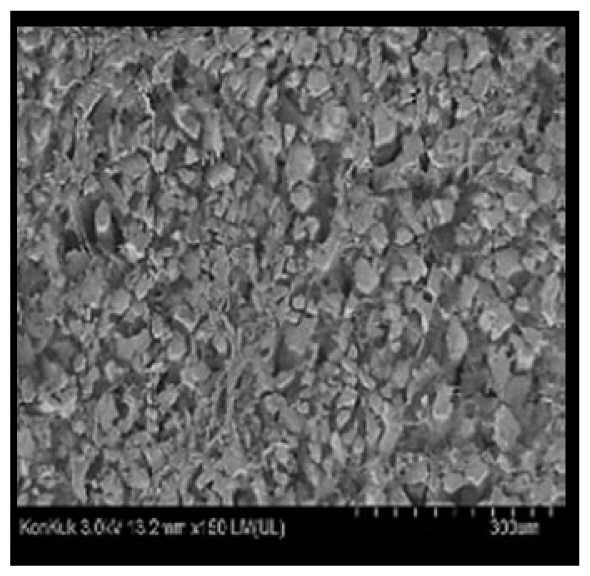
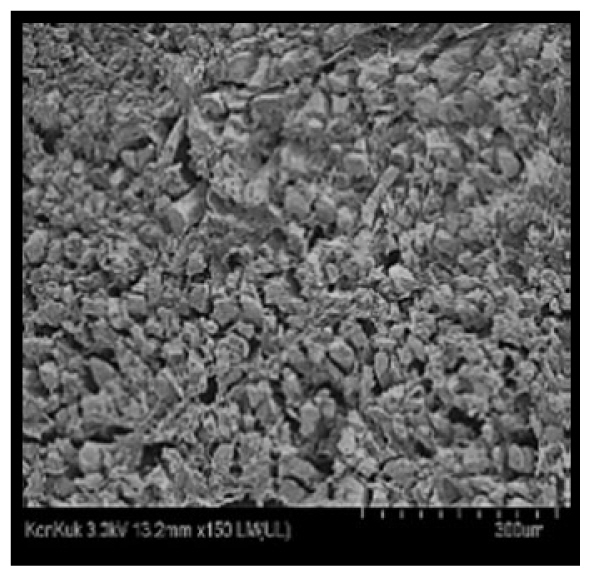
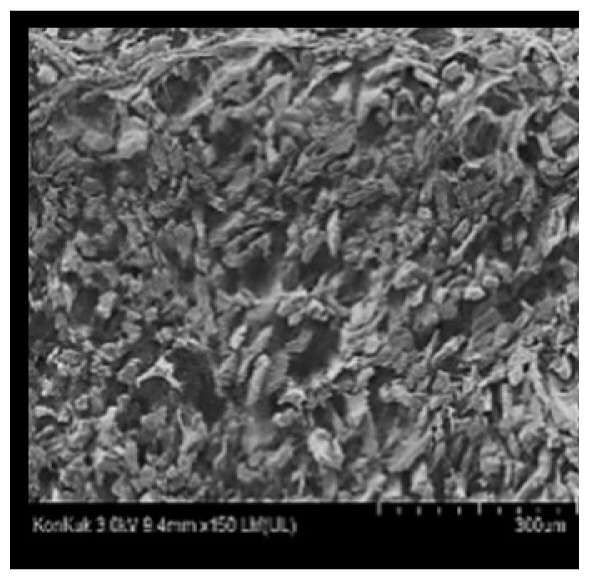
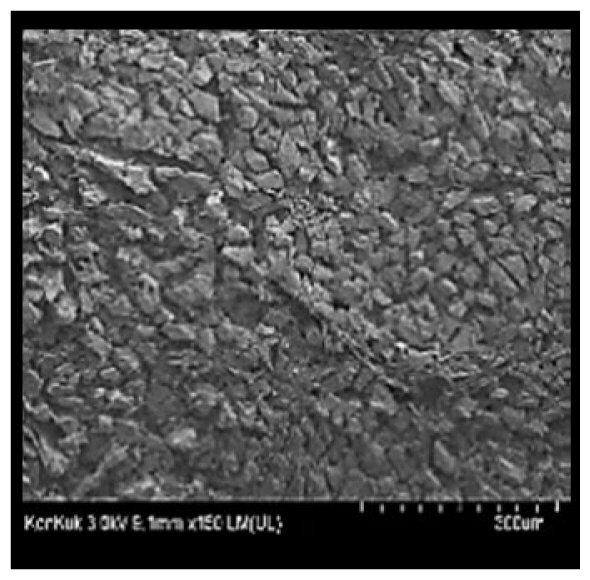
Cooked duck breast meat was cut into pieces with a dimension of 5×5×5 mm and freeze-dried at −40°C for 30 h. The freeze-dried samples were coated with platinum for 120 s. The section of the samples was scanned using a scanning electron microscope (Hitachi SU8010; HITACHI, Tokyo, Japan) at 3.0 kV, 8.8 mm×150 LM (UL) at 100× magnification.
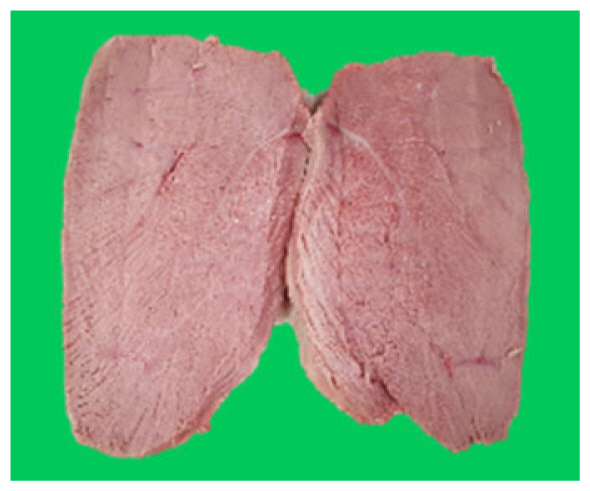
The results of the physicochemical and color analyses are shown in Table 1. Cooking loss in breast meat cooked using the sous-vide method ranged from 5.20% to 39.26%. Cooking loss in the samples cooked at 80°C for 180 min was significantly higher than that in the samples cooked at other temperatures for 60 or 180 min (p<0.05). The L*, b*, H°, and whiteness values increased, while the a* and C* values decreased with the increase in cooking time (Table 1). In this study, the cooking loss in the duck breast meat samples was directly proportional to the cooking temperature and time (p<0.05). Cooking loss is closely associated with lightness, shear force, and texture properties [12]. Decreasing cooking loss has economic benefits with respect to the meat industry as it increases the yield of the final product [13]. Approximately 80% of water in the muscle is trapped within the myofibrils [14]. The muscle fibers shrink and aggregate during the cooking process, which is attributed to the heat-induced muscle fiber damage. The deformation of muscle fiber decreases the physical space retaining free water in the meat and increases cooking loss [12]. Previous studies have reported that cooking loss increases with an increase in cooking temperature [15]. Consistent with this finding, this study demonstrated that cooking loss was high in meat cooked at high temperature for a prolonged time. Color is one of the most important quality attributes that affects consumer acceptance [16]. Duck meat is considered red meat due to its abundance of red muscle fibers [7]. The heat-induced denaturation of myoglobin can affect the color of red meat [17]. In the sous-vide method, the meat is processed at low temperatures, which prevents complete myoglobin denaturation. Hence, meat cooked using the sous-vide method maintains its red color. Additionally, the sous-vide method involves prolonged cooking time, which enables uniform cooking of the meat [16]. The high values of L* and whiteness, which are associated with light scattering, result from the aggregation and denaturation of myofibrillar proteins [15]. The a* value indicates the degree of myoglobin denaturation [18]. Myoglobin is a structural protein that also determines the color of the meat. Myoglobin begins to undergo denaturation at 60°C. The degree of myoglobin denaturation determines the redness of the cooked meat [19]. In this study, cooking at high temperatures for prolonged time decreased the a* values but increased the b* values of the cooked meat. The a* values may decrease due to protein denaturation, which promotes brown coloration and met-myoglobin formation [15]. The C* values, which measure brightness, ranged from 14.49 to 22.55 at the tested cooking temperatures. The C* values decreased with the increase in cooking temperature. At constant cooking temperature, the C* value decreased with the increase in cooking time. The H° value of cooked breast was the highest in the samples cooked at 80°C for 180 min (p<0.05). These results are consistent with previous studies, which reported that the H° value of the cooked products increases with the increase in cooking temperature and time [18,19].
The thiobarbituric acid reactive substance (TBARS) values are depicted in Figure 1. The TBARS values, which indicate the levels of lipid oxidation products, increased with the increase in cooking temperature at constant cooking time (p< 0.05). Similarly, the TBARS values increased with the increase in cooking time at a constant cooking temperature. Thus, the duck breast meat cooked using the sous-vide method at 80°C for 180 min exhibited the highest TBARS value. Lipid oxidation, which results from a complex free radical chain reaction, affects the rancidity of meat during storage [19]. The TBARS values of the duck breast reported in this study were higher than those reported for other meats, such as porcine or bovine. The TBARS value is dependent on the fatty acid composition of the duck fat [8]. The duck meat is rich in polyunsaturated fatty acid [20]. Although polyunsaturated fatty acid has various health benefits, it is susceptible to heat-induced oxidation [21]. In the sous-vide heating method, the duck meat is cooked at low temperatures (50°C to 60°C), which decreases the development of rancidity. This is consistent with the results of a previous study, which demonstrated that sous-vide cooking with low temperature could delay the increase in TBARS values [18].
The VBN content in the duck meat ranged from 14.89 to 19 mg% (Figure 1). The VBN content was not significantly different between the meat samples cooked at 60°C, 70°C, and 80°C, at both cooking times of 60 and 180 min. In contrast, the VBN contents in the duck breast meat cooked at 50°C for 60 or 180 min were markedly lower than those in the duck breast meat cooked at 60°C, 70°C, or 80°C. The VBN content is an indicator of protein deterioration in the meat [22]. VBN is one of the most important parameters to evaluate meat freshness [23]. The formation of volatile compounds is also influenced by cooking temperature and time [24]. Meat with a VBN value below 5 mg% is considered fresh, while values above 30 mg% indicate decomposition [25]. In this study, the VBN values of all the groups were within the acceptable range (14.89 to 19 mg%). This indicated that the sous-vide method does not promote protein deterioration in the duck meat.
The microbial counts of duck breast are shown in Table 2. The counts of TVC, E. coli, coliform, and LAB in the raw meat were 4.11, 3.95, 3.98, and 2.14 log CFU/g, respectively. No growth of psychrotrophic bacteria was observed in the raw meat. The microbial counts of all cooked samples, except for the sample cooked at 50°C, were below the detection limit (2 log CFU/g). The counts of total viable bacteria, E. coli, and coliform in the samples cooked at 50°C for 60 min were 3.89, 3.61, and 3.63 log CFU/g, respectively. Only the total viable bacteria (2.92 log CFU/g) were detected in the samples cooked at 50°C for 180 min. The cooking temperature and time can affect the microbial profile of the meat [15]. Cooking temperature can inhibit the growth of bacteria in the meat, especially when the heat energy is delivered to the meat core. Sous-vide cooking method effectively inhibits bacterial growth as the meat is uniformly cooked. The analysis of the cut surface of sous-vide-cooked meat indicated uniform heat transfer, which can affect bacterial growth. In this study, the sous-vide cooking method effectively inhibited the growth of bacteria [26].
The results of WBSF and TPA are shown in Table 3. Meat tenderness, which is measured based on the WBSF, is an important quality characteristic that influences consumer acceptance of meat products. The TPA is a crucial quality attribute of processed foods, as it affects their quality and palatability. The WBSF values, which indicate the force required to cut the meat, increased with longer cooking time and higher temperature. The WBSF value ranged from 1.89 to 6.96 kg, and different cooking conditions significantly affected the WBSF values of duck meat samples (p<0.05). Compared to the meat samples cooked at 50°C, the hardness was significantly higher in the meat samples cooked at 60°C, 70°C, or 80°C. Cooking time affected the hardness of the duck breast meat cooked at 50°C, and the duck breast meat cooked at 50°C for 180 min exhibited increased harness. The springiness, cohesiveness, chewiness and gumminess of the duck breast meat increased upon increasing the cooking temperature and time. The increase in cooking time and temperature resulting in increased WBSF and hardness may be attributed to the denaturation and aggregation of various proteins in duck meat. Most sarcoplasmic proteins aggregate between 40°C and 60°C, and heat aggregation of these proteins could extend up to 90°C [27]. Interestingly, when beef muscles are heated at low temperatures for a long time, sarcoplasmic proteins can undergo tenderization by some of enzymes. When myosin is hated above 65°C, some of the hydrophobic residues participate in protein─protein interactions to form gels for aggregation, while collagen and actin form aggregates between 65°C to 77°C, and between 80°C to 83°C, respectively [27]. Furthermore, heat treatment can cause myofibrils to shrink and water to be exuded from the muscle fibers. WBSF and texture profile are closely related to cooking loss, as meat texture is influenced by the content of immobilized water in the muscle fibers [28]. Preventing the denaturation of myofibrillar proteins can result in a softer texture and promote the retention of free water within the muscle fiber. Thus, the tenderness of sous-vide cooked duck breast can be influenced by factors such as water holding capacity, protein denaturation and aggregation, which depend on the specific cooking conditions.
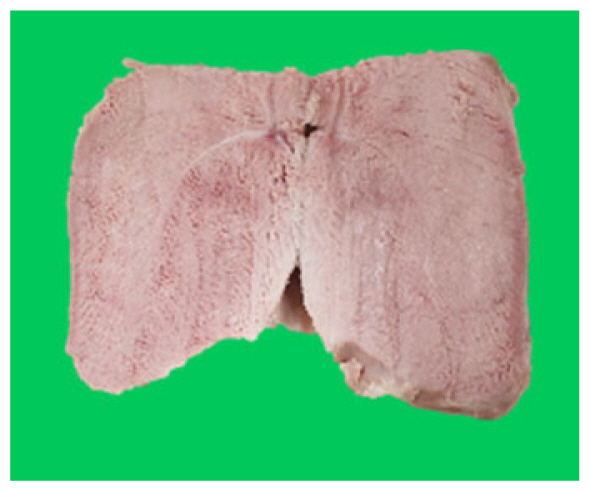
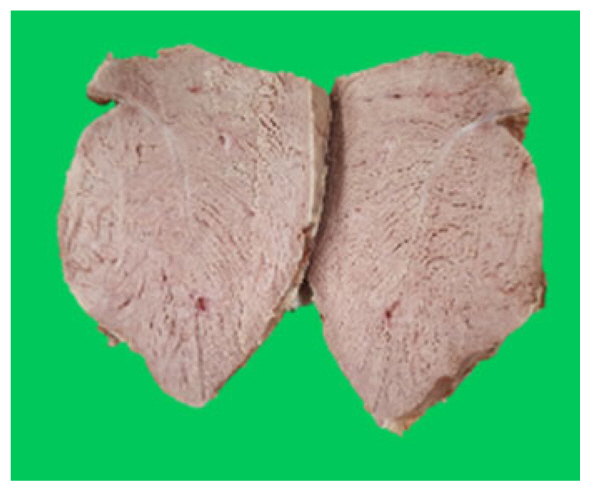
The microstructure of duck breast meat cooked using the sous-vide method is shown in Table 4. The myofibrils showed increased contraction with higher cooking temperatures and longer cooking times. Moreover, increasing the cooking temperature and time resulted in decreased redness of the samples and increased coarseness of the meat surface. Scanning electron microscopy (SEM) analysis showed that myofibril contraction and meat density increased with higher cooking temperature, while keeping the cooking time constant. In addition, increasing the cooking time, while keeping the cooking temperature constant, resulted in increased meat density. The pore size of the duck breast meat decreased with higher cooking temperatures and longer cooking times. The morphology of the duck breast meat exhibited consistency with its microstructure at different cooking temperatures and cooking times. The muscle tissue of the animal undergoes shrinkage and contraction upon heating, which is attributed to myofibrillar protein denaturation. The sarcomere length of the muscle fiber progressively decreases with higher cooking temperature [29]. Sarcomeres and myofibrils start shrinking at temperature between 40°C and 50°C, while the connective tissue and muscle fibers typically contract longitudinally at temperature between 60°C and 70°C [15]. Moreover, heating causes denaturation of myoglobin in the red meat, resulting in the loss of red color. The findings of this study revealed that the duck breast meat cooked using the sous-vide method showed myofibrillar shrinkage and discoloration mediated by myoglobin denaturation. SEM analysis showed that the meat structure density increased with higher cooking temperatures and longer cooking time, which can be attributed to the contraction of myofibrillar and connective tissues. This may explain the tenderness of the samples cooked at low temperatures.
This study evaluated the effect of sous-vide cooking temperature and time on cooking loss, color, texture, lipid oxidation, protein deterioration, and microstructure of the duck breast meat. The results of our study showed that cooking duck breast meat using sous-vide at a temperature of 60°C for at least 60 min resulted in acceptable tenderness and minimized rancidity. However, samples cooked at 50°C showed contamination with E. coli and coliforms. As a result, duck breast meat might be cooked at temperatures higher than 60°C for at least 60 min. The sous-vide cooking method helps maintain the quality attributes of the cooked duck breast meat, including texture and microbial safety. Additional studies are warranted to assess the effect of storage on the quality characteristics and sensory profiles of the duck breast cooked using the sous-vide method.
Notes
Figure 1
Evaluation of thiobarbituric acid reactive substance (TBARS) (a) and volatile basic nitrogen (VBN) (b) in the duck breast meat. The samples were cooked using the sous-vide method for 60 and 180 min at different temperatures (50°C, 60°C, 70°C, and 80°C). a–e Means with different letters are significantly different (p<0.05); values represent mean±standard deviation (n = 3).

Table 1
Physicochemical parameters of duck breast meat cooked using the sous-vide method
| Temperature (°C) | Time (min) | Cooking loss (%) | L*1) | a* | b* | Chroma | Hue° | Whiteness |
|---|---|---|---|---|---|---|---|---|
| 50 | 60 | 5.20±0.38e | 57.10±0.70e | 19.78±0.77a | 10.15±0.76c | 22.55±0.90a | 27.60±1.70e | 51.82±0.78e |
| 180 | 8.79±0.27de | 64.33±1.08c | 16.78±0.53b | 9.71±0.37c | 19.87±0.59bc | 31.96±1.22d | 59.18±0.45c | |
| 60 | 60 | 13.61±1.02d | 61.41±0.86d | 19.29±0.95a | 11.08±0.30b | 23.09±1.49a | 30.34±0.72d | 55.60±1.02d |
| 180 | 25.93±1.01b | 65.44±0.76b | 13.63±0.79d | 11.07±0.53b | 17.77±0.71d | 40.27±1.31c | 61.38±0.74b | |
| 70 | 60 | 19.91±1.47c | 67.46±1.39b | 15.64±1.21bc | 12.65±0.49a | 20.88±0.87b | 38.65±1.77c | 61.88±1.44b |
| 180 | 28.83±1.15b | 66.83±0.65a | 14.78±1.31cd | 11.35±0.62b | 18.52±0.89cd | 38.83±0.66c | 61.73±1.08ab | |
| 80 | 60 | 35.89±2.90a | 66.63±0.82a | 9.40±0.81e | 12.30±0.56a | 15.48±0.78e | 53.19±2.13b | 63.54±0.66a |
| 180 | 39.26±8.40a | 65.43±1.14a | 7.56±0.35f | 12.71±0.48a | 14.49±0.67e | 57.49±1.03a | 62.49±1.66ab |
Table 2
Microbial profile of duck breast meat cooked using the sous-vide method
| Temperature (°C) | Time (min) | Microorganism (log10 CFU/g) | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| TVB | E. coli | Coliform | LAB | Psychrophilic | ||
| Raw meat | 0 | 4.11±0.07a | 3.95±0.02a | 3.98±0.02a | 2.14±0.09 | N/D |
| 50 | 60 | 3.89±0.04b | 3.61±0.01b | 3.63±0.06b | N/D | N/D |
| 180 | 2.92±0.09c | N/D | N/D | N/D | N/D | |
| 60 | 60 | N/D | N/D | N/D | N/D | N/D |
| 180 | N/D | N/D | N/D | N/D | N/D | |
| 70 | 60 | N/D | N/D | N/D | N/D | N/D |
| 180 | N/D | N/D | N/D | N/D | N/D | |
| 80 | 60 | N/D | N/D | N/D | N/D | N/D |
| 180 | N/D | N/D | N/D | N/D | N/D | |
Table 3
Texture properties of duck breast meat cooked using the sous-vide method
| Temperature (°C) | Time (min) | WBSF (kg) | Hardness (kg) | Springiness | Cohesiveness | Chewiness (kg mm) | Gumminess (kg) |
|---|---|---|---|---|---|---|---|
| 50 | 60 | 1.89±0.08c | 0.52±0.07c | 0.56±0.06c | 0.39±0.04c | 0.13±0.03d | 0.25±0.03e |
| 180 | 2.47±0.18c | 0.77±0.15b | 0.57±0.03c | 0.41±0.03ab | 0.17±0.02d | 0.28±0.03e | |
| 60 | 60 | 2.76±0.60c | 1.13±0.13a | 0.58±0.05c | 0.41±0.05ab | 0.25±0.03c | 0.43±0.04d |
| 180 | 4.16±0.27bc | 1.19±0.18a | 0.60±0.05c | 0.45±0.05ab | 0.29±0.05c | 0.47±0.06cd | |
| 70 | 60 | 4.57±0.84bc | 1.25±0.97a | 0.62±0.03bc | 0.45±0.03ab | 0.35±0.03bc | 0.58±0.03ab |
| 180 | 5.19±0.27b | 1.20±0.85a | 0.67±0.10bc | 0.46±0.10ab | 0.38±0.05a | 0.50±0.05bc | |
| 80 | 60 | 4.79±0.45b | 1.22±0.65a | 0.80±0.07a | 0.53±0.07a | 0.39±0.05a | 0.56±0.03a |
| 180 | 6.96±0.67a | 1.28±0.67a | 0.72±0.10ab | 0.46±0.10b | 0.35±0.03ab | 0.49±0.02c |
REFERENCES
1. Yu TY, Morton JD, Clerens S, Dyer JM. Cooking-induced protein modifications in meat. Compr Rev Food Sci Food Saf 2017; 16:141–59.
https://doi.org/10.1111/1541-4337.12243


2. Ibarz A, Barbosa-Canovas GV. Introduction to food process engineering. Boca Raton, FL, USA: CRC Press; 2014. p. 722
https://doi.org/10.1201/b14969
3. Baldwin DE. Sous vide cooking: a review. Int J Gastron Food Sci 2012; 1:15–30.
https://doi.org/10.1016/j.ijgfs.2011.11.002

4. Schellekens M. New research issues in sous-vide cooking. Trends Food Sci Technol 1996; 7:256–62.
https://doi.org/10.1016/0924-2244(96)10027-3

5. Botinestean C, Keenan DF, Kerry JP, Hamill RM. The effect of thermal treatments including sous-vide, blast freezing and their combinations on beef tenderness of M. semitendinosus steaks targeted at elderly consumers. LWT-Food Sci Technol 2016; 74:154–9.
https://doi.org/10.1016/j.lwt.2016.07.026

6. Mortensen LM, Frøst MB, Skibsted LH, Risbo J. Effect of time and temperature on sensory properties in low-temperature long-time sous-vide cooking of beef. J Culinary Sci Technol 2012; 10:75–90.
https://doi.org/10.1080/15428052.2012.651024

7. Ali M, Kang GH, Yang HS, et al. A comparison of meat characteristics between duck and chicken breast. Asian-Australas J Anim Sci 2007; 20:1002–6.
https://doi.org/10.5713/ajas.2007.1002

8. Shin DM, Yune JH, Kim TK, et al. Physicochemical properties and oxidative stability of duck fat-added margarine for reducing the use of fully hydrogenated soybean oil. Food Chem 2021; 363:130260
https://doi.org/10.1016/j.foodchem.2021.130260


9. Kang ES, Kim HJ, Han SG, Seo HG. Duck oil-loaded nanoemulsion inhibits senescence of angiotensin II-treated vascular smooth muscle cells by upregulating SIRT1. Food Sci Anim Resour 2020; 40:106–17.
https://doi.org/10.5851/kosfa.2019.e93



10. Wang B, Zhang M, Ge W, He K, Cheng F. Microencapsulated duck oil diacylglycerol: Preparation and application as anti-obesity agent. LWT-Food Sci Technol 2019; 101:646–52.
https://doi.org/10.1016/j.lwt.2018.11.061

11. Shin DM, Hwang KE, Lee CW, Kim TK, Park YS, Han SG. Effect of Swiss chard (Beta vulgaris var. cicla) as nitrite replacement on color stability and shelf-life of cooked pork patties during refrigerated storage. Korean J Food Sci Anim Resour 2017; 37:418–28.
https://doi.org/10.5851/kosfa.2017.37.3.418



12. del Pulgar JS, Gázquez A, Ruiz-Carrascal J. Physico-chemical, textural and structural characteristics of sous-vide cooked pork cheeks as affected by vacuum, cooking temperature, and cooking time. Meat Sci 2012; 90:828–35.
https://doi.org/10.1016/j.meatsci.2011.11.024


13. Dominguez-Hernandez E, Salaseviciene A, Ertbjerg P. Low-temperature long-time cooking of meat: Eating quality and underlying mechanisms. Meat Sci 2018; 143:104–13.
https://doi.org/10.1016/j.meatsci.2018.04.032


14. Offer G, Knight P, Jeacocke R, et al. The structural basis of the water-holding, appearance and toughness of meat and meat products. Food Struct 1989; 8:17
15. Roldán M, Antequera T, Martín A, Mayoral AI, Ruiz J. Effect of different temperature–time combinations on physicochemical, microbiological, textural and structural features of sous-vide cooked lamb loins. Meat Sci 2013; 93:572–8.
https://doi.org/10.1016/j.meatsci.2012.11.014


16. Ayub H, Ahmad A. Physiochemical changes in sous-vide and conventionally cooked meat. Int J Gastron Food Sci 2019; 17:100145
https://doi.org/10.1016/j.ijgfs.2019.100145

17. Oz F, Aksu MI, Turan M. The effects of different cooking methods on some quality criteria and mineral composition of beef steaks. J Food Process Preserv 2017; 41:e13008
https://doi.org/10.1111/jfpp.13008

18. Jeong K, Hyeonbin O, Shin SY, Kim YS. Effects of sous-vide method at different temperatures, times and vacuum degrees on the quality, structural, and microbiological properties of pork ham. Meat Sci 2018; 143:1–7.
https://doi.org/10.1016/j.meatsci.2018.04.010


19. Bhat ZF, Morton JD, Zhang X, Mason SL, Bekhit AEDA. Sous-vide cooking improves the quality and in-vitro digestibility of Semitendinosus from culled dairy cows. Food Res Int 2020; 127:108708
https://doi.org/10.1016/j.foodres.2019.108708


20. Shin DM, Kim DH, Yune JH, et al. Oxidative stability and quality characteristics of duck, chicken, swine and bovine skin fats extracted by pressurized hot water extraction. Food Sci Anim Resour 2019; 39:446–58.
https://doi.org/10.5851/kosfa.2019.e41



21. Ladikos D, Lougovois V. Lipid oxidation in muscle foods: a review. Food Chem 1990; 35:295–314.
https://doi.org/10.1016/0308-8146(90)90019-Z

22. Min JS, Lee SO, Jang A, Jo C, Park CS, Lee M. Relationship between the concentration of biogenic amines and volatile basic nitrogen in fresh beef, pork, and chicken meat. Asian-Australas J Anim Sci 2007; 20:1278–84.
https://doi.org/10.5713/ajas.2007.1278

23. Hong GE, Kim JH, Ahn SJ, Lee CH. Changes in meat quality characteristics of the sous-vide cooked chicken breast during refrigerated storage. Korean J Food Sci Anim Resour 2015; 35:757–64.
https://doi.org/10.5851/kosfa.2015.35.6.757



24. Choi YS, Kim JH, Park BY, Lee JM, Kim IS, Kim BC. Physicochemical, microbiological and sensory properties of Korean frozen pork loins for export. J Anim Sci Technol 2002; 44:361–8.
https://doi.org/10.5187/JAST.2002.44.3.361

25. Roldán M, Ruiz J, del Pulgar JS, Pérez-Palacios T, Antequera T. Volatile compound profile of sous-vide cooked lamb loins at different temperature–time combinations. Meat Sci 2015; 100:52–7.
https://doi.org/10.1016/j.meatsci.2014.09.010


26. Dogruyol H, Mol S, Cosansu S. Increased thermal sensitivity of Listeria monocytogenes in sous-vide salmon by oregano essential oil and citric acid. Food Microbiol 2020; 90:103496
https://doi.org/10.1016/j.fm.2020.103496


27. Tornberg E. Effects of heat on meat proteins–Implications on structure and quality of meat products. Meat Sci 2005; 70:493–508.
https://doi.org/10.1016/j.meatsci.2004.11.021


28. Aktaş N, Aksu MI, Kaya M. The effect of organic acid marination on tenderness, cooking loss and bound water content of beef. J Muscle Foods 2003; 14:181–94.
https://doi.org/10.1111/j.1745-4573.2003.tb00699.x

29. Souza CM, Boler DD, Clark DL, et al. The effects of high pressure processing on pork quality, palatability, and further processed products. Meat Sci 2011; 87:419–27.
https://doi.org/10.1016/j.meatsci.2010.11.023


- TOOLS
-
METRICS

- Related articles
-
Effect of shearing on some physiological and hormonal parameters in Akkaraman sheep 2020 May;33(5)











 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print




