 |
 |
| Anim Biosci > Volume 36(12); 2023 > Article |
|
Abstract
Objective
Methods
Results
Notes
CONFLICT OF INTEREST
We certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
FUNDING
This work was supported by funds from the National Natural Science Foundation of China (31660682), the Yunnan Program for Key Research and Development Project (2018BB002-02), The technical Innovation Talents of Yunnan Province (2018HB075), and the Key Project of Agricultural Joint Fund of Yunnan Province (202101BD070001-026).
Figure 1

Figure 2
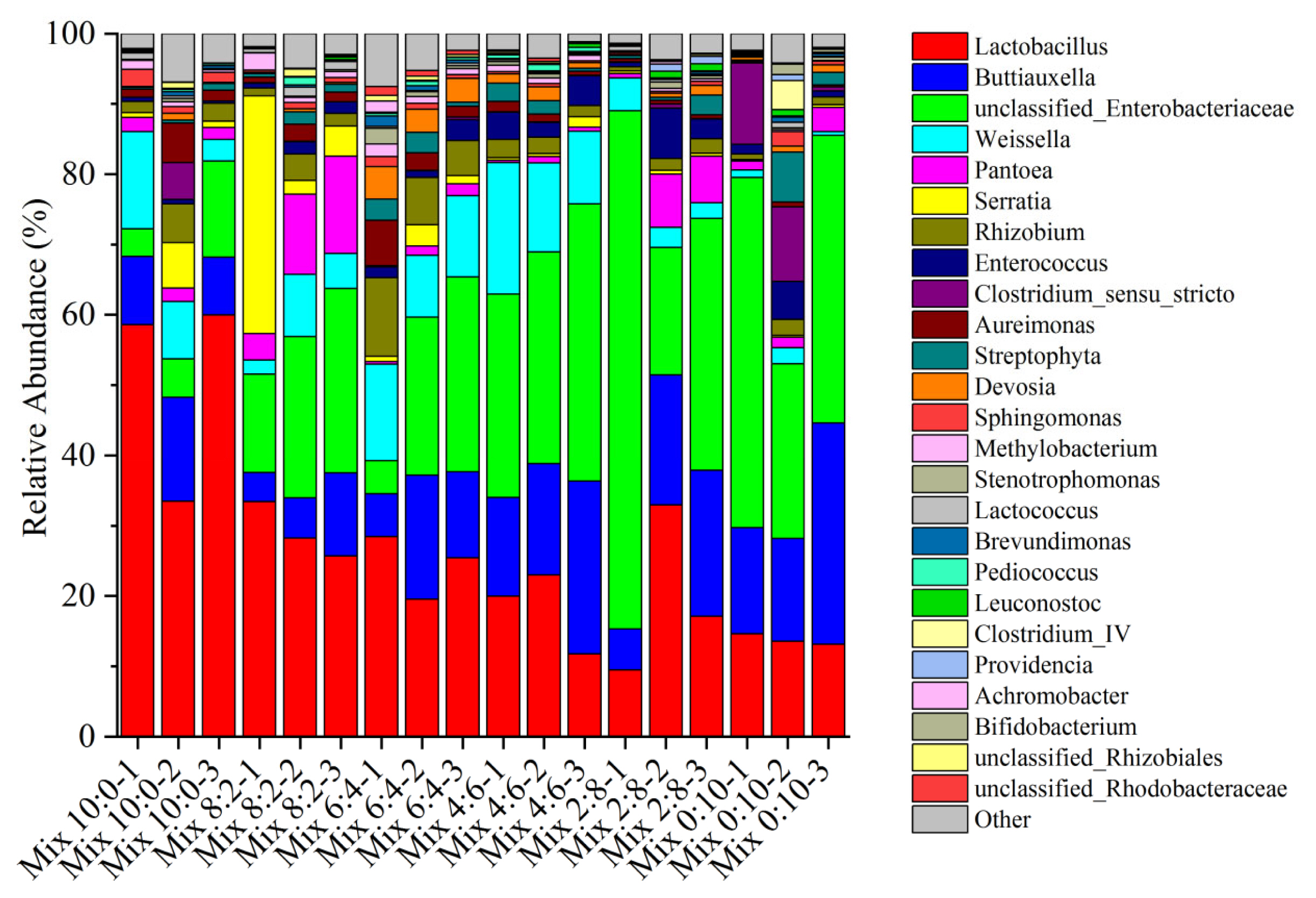
Figure 3
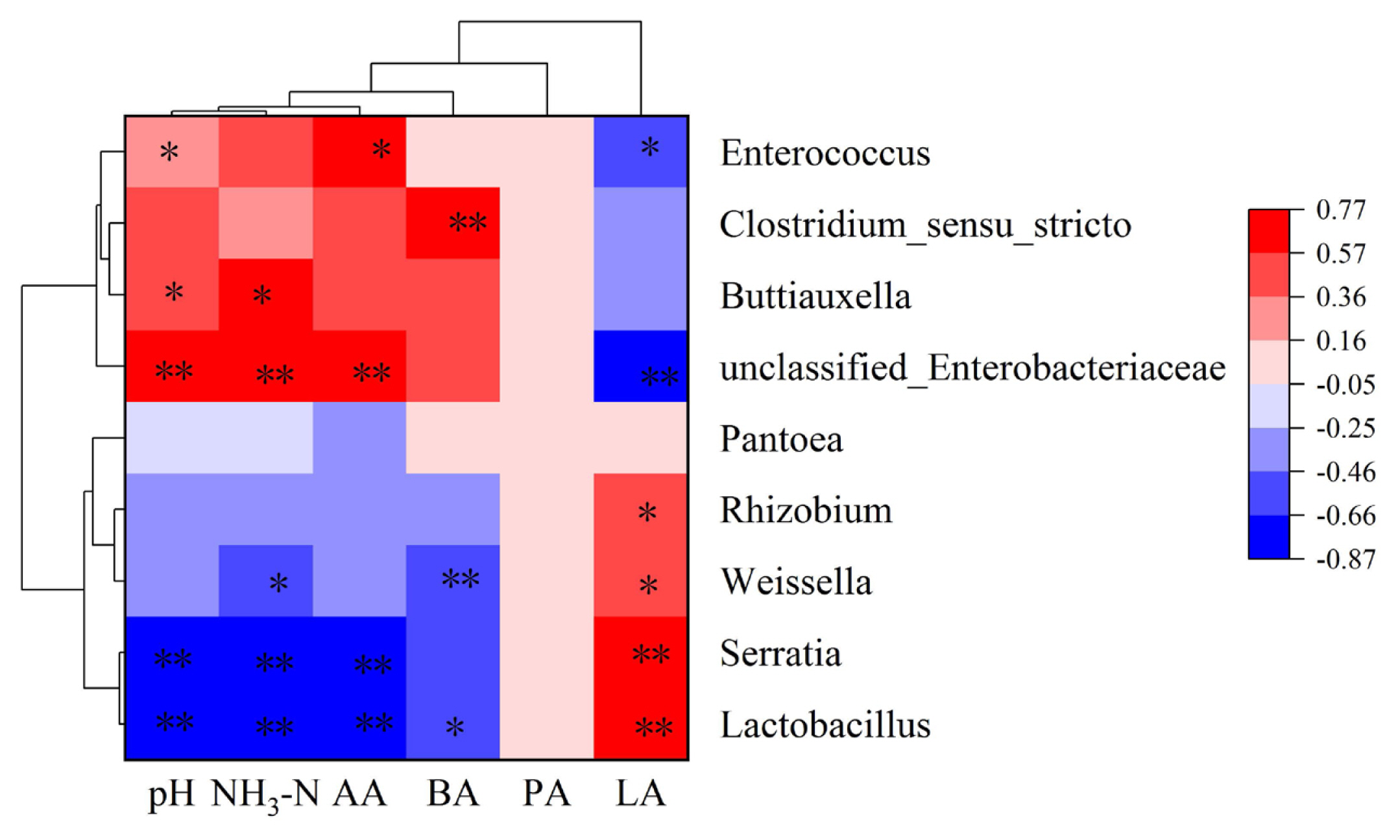
Table 1
| Item | Treatment1) | SEM | p-value2) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Corn | Mix 8:2 | Mix 6:4 | Mix 4:6 | Mix 2:8 | Hairy vetch | T | L | Q | ||
| Chemical composition | ||||||||||
| DM (g/kg FM) | 300a | 283ab | 266b | 248c | 232cd | 216d | 7.24 | <0.01 | <0.01 | 0.87 |
| WSC (g/kg DM) | 105a | 90.1b | 77.5b | 61.2c | 47.4d | 32.9e | 6.76 | <0.01 | <0.01 | 0.20 |
| CP (g/kg DM) | 51.7f | 77.7e | 94.0d | 132c | 162b | 202a | 11.7 | <0.01 | <0.01 | <0.01 |
| NDF (g/kg DM) | 522a | 521a | 500a | 508a | 539a | 569a | 31.4 | 0.51 | 0.65 | 0.40 |
| ADF g/kg DM | 242a | 240a | 253a | 230a | 238a | 243a | 4.73 | 0.88 | 0.83 | 0.91 |
| Hemicellulose | 280a | 286a | 247a | 278a | 301a | 327a | 11.7 | 0.57 | 0.24 | 0.23 |
| EE (g/kg DM) | 14.3d | 16.1d | 17.2cd | 21.4bc | 24.0ab | 26.4a | 1.74 | <0.01 | <0.01 | 0.53 |
| BC (mEq/kg DM) | 34.7f | 43.6e | 48.4d | 55.0c | 65.5b | 70.7a | 3.02 | <0.01 | <0.01 | 0.65 |
| Microorganism log10 cfu/g of FM | ||||||||||
| LAB | 6.55a | 6.11b | 5.61c | 5.34d | 4.91d | 4.54e | 0.17 | <0.01 | <0.01 | 0.12 |
| Aerobic bacteria | 5.68a | 5.60a | 5.46a | 5.60a | 5.66a | 5.65a | 0.03 | 0.44 | 0.79 | 0.15 |
| Yeast | 5.28a | 5.10a | 4.83b | 4.59c | 4.60c | 4.32d | 0.08 | <0.01 | <0.01 | 0.37 |
| Mold | 4.65c | 4.97bc | 5.09abc | 5.18ab | 5.49ab | 5.52 | 0.09 | 0.02 | <0.01 | 0.66 |
SEM, standard error of the means; DM, dry matter; FM, fresh matter; WSC, water-soluble carbohydrates; CP, crude protein; NDF, neutral detergent fiber assayed with a heat stable amylase and expressed inclusive of residual ash; ADF, acid detergent fiber expressed inclusive of residual ash; EE, ether extract; BC, buffering capacity; LAB, lactic acid bacteria; CFU, colony-forming units.
Table 2
| Item | Treatment1) | SEM | p-value2) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Mix 10:0 | Mix 8:2 | Mix 6:4 | Mix 4:6 | Mix 2:8 | Mix 0:10 | T | L | Q | ||
| pH | 3.95e | 4.10d | 4.24c | 4.34b | 4.68b | 5.11a | 0.94 | <0.01 | <0.01 | <0.01 |
| NH3-N (g/kg) | 20.9f | 32.5e | 42.9d | 51.8c | 67.5b | 83.2a | 0.27 | <0.01 | <0.01 | <0.01 |
| LA (g/kg DM) | 81.0a | 69.9b | 78.7a | 65.2c | 54.9d | 21.4e | 1.05 | <0.01 | <0.01 | <0.01 |
| AA (g/kg DM) | 13.2d | 14.5c | 15.7c | 25.9b | 25.7b | 33.8a | 2.27 | <0.01 | <0.01 | <0.01 |
| PA (g/kg DM) | ND | ND | ND | ND | ND | ND | - | - | - | - |
| BA (g/kg DM) | ND | ND | ND | ND | ND | 5.48 | - | - | - | - |
SEM, standard error of the means; NH3-N, ammonia-N; LA, lactic acid; AA, acetic acid; PA, propionic acid; BA, butyric acid; ND, not detected.
Table 3
| Item | Treatment1) | SEM | p-value2) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Mix 10:0 | Mix 8:2 | Mix 6:4 | Mix 4:6 | Mix 2:8 | Mix 0:10 | T | L | Q | ||
| DM (g/kg FM) | 286a | 272a | 267b | 252c | 229d | 184e | 1.06 | <0.01 | <0.01 | <0.01 |
| WSC (g/kg DM) | 62.4a | 47.1c | 39.7d | 35.3d | 21.3e | 18.6e | 3.72 | <0.01 | <0.01 | 0.09 |
| CP (g/kg DM) | 56.1f | 75.1e | 97.3d | 127c | 156b | 183a | 1.08 | <0.01 | <0.01 | 0.04 |
| NDF (g/kg DM) | 612a | 604a | 560b | 485c | 468c | 465c | 1.55 | <0.01 | <0.01 | 0.27 |
| ADF (g/kg DM) | 315a | 308a | 298a | 282a | 278a | 261a | 0.98 | 0.69 | 0.14 | 0.80 |
| Hemicellulose | 297a | 296a | 262ab | 203b | 190b | 200b | 1.41 | <0.01 | <0.01 | 0.43 |
| EE (g/kg DM) | 12.7c | 15.9c | 22.8b | 28.9ab | 26.7b | 33.1a | 1.85 | <0.01 | <0.01 | 0.28 |
SEM, standard error of the means; DM, dry matter; FM, fresh matter; WSC, water-soluble carbohydrates; CP, crude protein; NDF, neutral detergent fiber; ADF, acid detergent fiber expressed inclusive of residual ash; EE, ether extract.
Table 4
| Item | Treatment1) | SEM | p-value2) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Mix 10:0 | Mix 8:2 | Mix 6:4 | Mix 4:6 | Mix 2:8 | Mix 0:10 | T | L | Q | ||
| LAB (log10 cfu/g of FM) | 7.12a | 6.91b | 6.73c | 6.29d | 6.29d | 5.80e | 0.08 | <0.01 | 0.04 | 0.17 |
| Aerobic bacteria (log10 cfu/g of FM) | 5.56d | 5.87c | 6.19b | 7.12a | 7.17a | 7.29a | 0.08 | <0.01 | <0.01 | <0.01 |
| Yeast (log10 cfu/g of FM) | 1.78b | 1.26c | 1.04c | ND | ND | 3.23a | 0.10 | <0.01 | <0.01 | <0.01 |
| Mold (log10 cfu/g of FM) | ND | ND | ND | ND | ND | ND | - | - | - | - |
SEM, standard error of the means; LAB, lactic acid bacteria; CFU, colony-forming units; FM, fresh matter; ND, not detected.
Table 5
| Item | Treatment1) | SEM | p-value2) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Mix 10:0 | Mix 8:2 | Mix 6:4 | Mix 4:6 | Mix 2:8 | Mix 0:10 | T | L | Q | ||
| Read | 40,811a | 43,731a | 44,357a | 45,288a | 45,824a | 43,728a | 1,125 | 0.89 | 0.40 | 0.45 |
| OTU number | 217b | 225b | 268a | 228b | 224b | 201b | 6.15 | <0.01 | 0.14 | <0.01 |
| Ace | 284ab | 291a | 293a | 277ab | 273ab | 243b | 6.31 | 0.04 | 0.19 | 0.14 |
| Chao | 271c | 296ab | 310a | 248d | 274bc | 244d | 7.29 | <0.01 | 0.15 | 0.43 |
| Shannon | 2.29b | 2.63b | 3.17a | 2.30b | 2.49b | 2.00c | 0.10 | <0.01 | 0.35 | 0.75 |
| Simpson | 0.13bc | 0.15bc | 0.11c | 0.15bc | 0.18b | 0.25a | 0.17 | 0.04 | 0.22 | 0.82 |
| Coverage | 99.9 | 99.9 | 99.9 | 99.9 | 99.9 | 99.9 | - | - | - | - |
REFERENCES
- TOOLS






 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print





